Expression of Tgfβ1 and Inflammatory Markers in the 6-hydroxydopamine Mouse Model of Parkinson's Disease
- PMID: 26869879
- PMCID: PMC4737885
- DOI: 10.3389/fnmol.2016.00007
Expression of Tgfβ1 and Inflammatory Markers in the 6-hydroxydopamine Mouse Model of Parkinson's Disease
Abstract
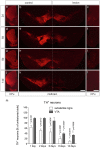
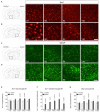
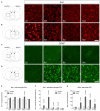
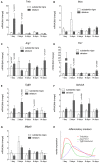
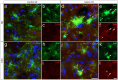
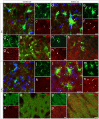
Parkinson's disease (PD) is a neurodegenerative disorder that is characterized by loss of midbrain dopaminergic (mDA) neurons in the substantia nigra (SN). Microglia-mediated neuroinflammation has been described as a common hallmark of PD and is believed to further trigger the progression of neurodegenerative events. Injections of 6-hydroxydopamine (6-OHDA) are widely used to induce degeneration of mDA neurons in rodents as an attempt to mimic PD and to study neurodegeneration, neuroinflammation as well as potential therapeutic approaches. In the present study, we addressed microglia and astroglia reactivity in the SN and the caudatoputamen (CPu) after 6-OHDA injections into the medial forebrain bundle (MFB), and further analyzed the temporal and spatial expression patterns of pro-inflammatory and anti-inflammatory markers in this mouse model of PD. We provide evidence that activated microglia as well as neurons in the lesioned SN and CPu express Transforming growth factor β1 (Tgfβ1), which overlaps with the downregulation of pro-inflammatory markers Tnfα, and iNos, and upregulation of anti-inflammatory markers Ym1 and Arg1. Taken together, the data presented in this study suggest an important role for Tgfβ1 as a lesion-associated factor that might be involved in regulating microglia activation states in the 6-OHDA mouse model of PD in order to prevent degeneration of uninjured neurons by microglia-mediated release of neurotoxic factors such as Tnfα and nitric oxide (NO).
Keywords: 6-OHDA; Tgfβ1; Tnfα; astrocytes; microglia.
Figures
References
-
- Bortolanza M., Cavalcanti-Kiwiatkowski R., Padovan-Neto F. E., da Silva C. A., Mitkovski M., Raisman-Vozari R., et al. (2014). Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol. Dis. 73, 377–387. 10.1016/j.nbd.2014.10.017 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

