Syndecans as Cell Surface Receptors in Cancer Biology. A Focus on their Interaction with PDZ Domain Proteins
- PMID: 26869927
- PMCID: PMC4735372
- DOI: 10.3389/fphar.2016.00010
Syndecans as Cell Surface Receptors in Cancer Biology. A Focus on their Interaction with PDZ Domain Proteins
Abstract
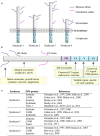
Syndecans are transmembrane receptors with ectodomains that are modified by glycosaminoglycan chains. The ectodomains can interact with a wide variety of molecules, including growth factors, cytokines, proteinases, adhesion receptors, and extracellular matrix (ECM) components. The four syndecans in mammals are expressed in a development-, cell-type-, and tissue-specific manner and can function either as co-receptors with other cell surface receptors or as independent adhesion receptors that mediate cell signaling. They help regulate cell proliferation and migration, angiogenesis, cell/cell and cell/ECM adhesion, and they may participate in several key tumorigenesis processes. In some cancers, syndecan expression regulates tumor cell proliferation, adhesion, motility, and other functions, and may be a prognostic marker for tumor progression and patient survival. The short cytoplasmic tail is likely to be involved in these events through recruitment of signaling partners. In particular, the conserved carboxyl-terminal EFYA tetrapeptide sequence that is present in all syndecans binds to some PDZ domain-containing proteins that may function as scaffold proteins that recruit signaling and cytoskeletal proteins to the plasma membrane. There is growing interest in understanding these interactions at both the structural and biological levels, and recent findings show their high degree of complexity. Parameters that influence the recruitment of PDZ domain proteins by syndecans, such as binding specificity and affinity, are the focus of active investigations and are important for understanding regulatory mechanisms. Recent studies show that binding may be affected by post-translational events that influence regulatory mechanisms, such as phosphorylation within the syndecan cytoplasmic tail.
Keywords: PDZ domain; cancer; cytoskeleton; extracellular matrix; phosphorylation; syndecan.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

