Resveratrol suppresses human glioblastoma cell migration and invasion via activation of RhoA/ROCK signaling pathway
- PMID: 26870238
- PMCID: PMC4727060
- DOI: 10.3892/ol.2015.3888
Resveratrol suppresses human glioblastoma cell migration and invasion via activation of RhoA/ROCK signaling pathway
Abstract
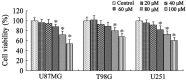
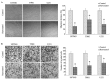
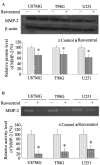
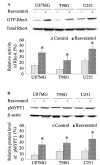
Numerous studies have demonstrated that resveratrol has a potential use in cancer prevention and treatment. However, the effects of resveratrol on cancer cell motility and invasiveness remain unclear. The current study aimed to examine the effects of resveratrol on cell migration and invasion in human glioblastoma cells, and to explore the underlying molecular mechanisms. In wound-healing and Matrigel transwell assays, resveratrol was found to significantly inhibit the migration and invasion of U87MG, T98G and U251 glioblastoma cells in vitro. Results from western blot analysis and gelatin zymography revealed that resveratrol also suppressed the expression and activity of matrix metalloproteinase 2 (MMP-2; P<0.05), an important mediator of cell migration and invasion. Furthermore, using a pull-down assay, increased activation of RhoA was observed in glioblastoma cells treated with resveratrol vs. controls (P<0.05). Notably, inhibition of the RhoA/Rho-associated kinase (ROCK) pathway by C3 transferase or Y-27362 was found to attenuate the resveratrol-induced reductions in cell migration and invasion (P<0.05), and also partially rescued the decreased expression and activity of MMP-2 induced by resveratrol (P<0.05). Taken together, the results suggest that resveratrol may inhibit glioblastoma cell motility and invasiveness via activating the RhoA/ROCK signaling pathway.
Keywords: RhoA; glioblastoma; invasion; migration; resveratrol.
Figures

Similar articles
-
PDIA6 regulation of ADAM17 shedding activity and EGFR-mediated migration and invasion of glioblastoma cells.J Neurosurg. 2017 Jun;126(6):1829-1838. doi: 10.3171/2016.5.JNS152831. Epub 2016 Aug 19. J Neurosurg. 2017. PMID: 27540907
-
PEITC inhibits human brain glioblastoma GBM 8401 cell migration and invasion through the inhibition of uPA, Rho A, and Ras with inhibition of MMP-2, -7 and -9 gene expression.Oncol Rep. 2015 Nov;34(5):2489-96. doi: 10.3892/or.2015.4260. Epub 2015 Sep 8. Oncol Rep. 2015. PMID: 26352173
-
Paeoniflorin Inhibits Hepatocyte Growth Factor- (HGF-) Induced Migration and Invasion and Actin Rearrangement via Suppression of c-Met-Mediated RhoA/ROCK Signaling in Glioblastoma.Biomed Res Int. 2019 Feb 11;2019:9053295. doi: 10.1155/2019/9053295. eCollection 2019. Biomed Res Int. 2019. PMID: 30886866 Free PMC article.
-
Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells.J Oral Pathol Med. 2015 Oct;44(9):699-706. doi: 10.1111/jop.12288. Epub 2014 Nov 17. J Oral Pathol Med. 2015. PMID: 25401496
-
Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways.Int J Oncol. 2016 Aug;49(2):735-43. doi: 10.3892/ijo.2016.3559. Epub 2016 Jun 3. Int J Oncol. 2016. PMID: 27278736
Cited by
-
Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms.Cancers (Basel). 2024 Sep 19;16(18):3193. doi: 10.3390/cancers16183193. Cancers (Basel). 2024. PMID: 39335164 Free PMC article. Review.
-
Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis.Exp Mol Med. 2017 Feb 24;49(2):e296. doi: 10.1038/emm.2016.151. Exp Mol Med. 2017. PMID: 28232662 Free PMC article.
-
Rnd3 Is a Crucial Mediator of the Invasive Phenotype of Glioblastoma Cells Downstream of Receptor Tyrosine Kinase Signalling.Cells. 2022 Nov 22;11(23):3716. doi: 10.3390/cells11233716. Cells. 2022. PMID: 36496976 Free PMC article.
-
The Role of Rho GTPases in Motility and Invasion of Glioblastoma Cells.Anal Cell Pathol (Amst). 2020 Jan 31;2020:9274016. doi: 10.1155/2020/9274016. eCollection 2020. Anal Cell Pathol (Amst). 2020. PMID: 32089990 Free PMC article. Review.
-
The role of abnormal epigenetic regulation of small GTPases in glioma (Review).Int J Oncol. 2025 Aug;67(2):63. doi: 10.3892/ijo.2025.5769. Epub 2025 Jul 4. Int J Oncol. 2025. PMID: 40613212 Free PMC article. Review.
References
-
- Taylor JW, Chi AS, Cahill DP. Tailored therapy in diffuse gliomas: Using molecular classifiers to optimize clinical management. Oncology (Williston Park) 2013;27:504–514. - PubMed
-
- Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
