The Cytoplasmic and Periplasmic Expression Levels and Folding of Organophosphorus Hydrolase Enzyme in Escherichia coli
- PMID: 26870308
- PMCID: PMC4746795
- DOI: 10.5812/jjm.17790
The Cytoplasmic and Periplasmic Expression Levels and Folding of Organophosphorus Hydrolase Enzyme in Escherichia coli
Abstract
Background: Organophosphorus hydrolase (OPH) is a type of organophosphate-degrading enzyme which is widely used in the bioremediation process.
Objectives: In this study, the periplasmic and cytoplasmic productions and the activity of recombinant OPH in Escherichia coli were investigated and compared using two pET systems (pET21a and pET26b).
Materials and methods: The sequence encoding the opd gene was synthesized and expressed in the form of inclusion body using pET21a-opd and in the periplasmic space in pET26b-opd.
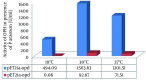
Results: Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed a band of about 37 kDa with a maximum expression level at 30°C from pET21a-opd.However, the obtained results of the periplasmic space extraction of OPH (pET26b-opd) showed a very weak band, while the cytoplasmic expression of OPH (pET21a-opd) produced a strong protein band.
Conclusions: The activities studied by the production of PNP were determined by following the increase at 410 nm. The maximum PNP was produced at 30°C with an optical density of 10.62 in the presence of cytoplasmic expression of OPH (pET21a-opd). Consequently, our results suggest cytoplasmic expression system as an appropriate candidate with a high amount of OPH in spite of inclusion body formation, which needs an additional refolding step.
Keywords: Cytoplasmic; Escherichia coli; Organophosphorus; Periplasmic.
Figures


References
-
- Bardin PG, van Eeden SF, Moolman JA, Foden AP, Joubert JR. Organophosphate and carbamate poisoning. Arch Intern Med. 1994;154(13):1433–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
