Crystal structures of two 2,9-di-thia-13-aza-dispiro-[4.1.4(7).3(5)]tetra-decan-6-ones
- PMID: 26870418
- PMCID: PMC4719827
- DOI: 10.1107/S2056989015020885
Crystal structures of two 2,9-di-thia-13-aza-dispiro-[4.1.4(7).3(5)]tetra-decan-6-ones
Abstract
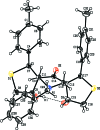
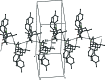
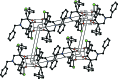
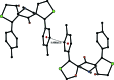
In the title compounds 4,11-dihy-droxy-13-methyl-1,8-di-p-tolyl-2,9-di-thia-13- aza-dispiro-[4.1.4(7).3(5)]tetra-decan-6-one, C26H31NO3S2, (I), and 13-benzyl-4,11-dihy-droxy-1,8-bis-(4-methyl-phen-yl)-2,9-di-thia-13-aza-dispiro-[4.1.4(7).3(5)]tetradecan-6-one, C32H35NO3S2, (II), the piperidine rings adopt distorted chair conformations. The thio-phene rings in (I) have envelope conformations, with the spiro C atoms as the flaps. In (II), one thio-phene ring (D) has an envelope conformation, with the hy-droxy-substituted C atom as the flap, while the other thio-phene ring (E) has a twisted conformation on the C-C bond involving the spiro C atom and the toluyl-substituted C atom. In (I), the mean plane of the piperidine ring makes dihedral angles of 75.16 (9) and 73.33 (8)° with the mean planes of the thio-phene rings (D and E), respectively. In (II), the corresponding dihedral angles are 70.95 (11) and 77.43 (12)°. In both compounds, there is an intra-molecular O-H⋯O hydrogen bond forming an S(6) ring motif. In the crystal of (I), mol-ecules are linked via O-H⋯N and C-H⋯O hydrogen bonds, forming chains along [010]. There are also π-π inter-actions present involving inversion-related benzene rings, linking the chains to form slabs parallel to (100). In the crystal of (II), mol-ecules are linked via O-H⋯O hydrogen bonds, forming inversion dimers with an R 4 (4)(8) ring motif. The dimers are linked by C-H⋯π inter-actions, forming slabs parallel to (001).
Keywords: 2,9-dithia-13-azadispiro[4.1.47.35]]tetradecan-6-one; crystal structure; dispiro: hydrogen bonding; piperidine; thiophene.
Figures



Similar articles
-
13-Benzyl-4,11-dihy-droxy-1,8-diphen-yl-2,9-di-thia-13-aza-dispiro-[4.1.4.3]tetra-decan-6-one.IUCrdata. 2021 Feb 26;6(Pt 2):x210210. doi: 10.1107/S2414314621002108. eCollection 2021 Feb. IUCrdata. 2021. PMID: 36338860 Free PMC article.
-
Crystal structures of ethyl (2S*,2'R*)-1'-methyl-2'',3-dioxo-2,3-di-hydro-dispiro-[1-benzo-thio-phene-2,3'-pyrrolidine-2',3''-indoline]-4'-carboxyl-ate and ethyl (2S*,2'R*)-5''-chloro-1'-methyl-2'',3-dioxo-2,3-di-hydro-dispiro-[1-benzo-thio-phene-2,3'-pyrrolidine-2',3''-indoline]-4'-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2014 Jul 19;70(Pt 8):94-7. doi: 10.1107/S1600536814015426. eCollection 2014 Aug 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 25249864 Free PMC article.
-
Crystal structures of 6a,6b,7,11a-tetra-hydro-6H,9H-spiro-[chromeno[3',4':3,4]pyrrolo-[1,2-c]thia-zole-11,3'-indoline]-2',6-dione and 5'-methyl-6a,6b,7,11a-tetra-hydro-6H,9H-spiro-[chromeno[3',4':3,4]pyrrolo-[1,2-c]thia-zole-11,3'-indoline]-2',6-dione.Acta Crystallogr E Crystallogr Commun. 2019 Jan 22;75(Pt 2):246-250. doi: 10.1107/S2056989019000045. eCollection 2019 Feb 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 30800460 Free PMC article.
-
14-Hy-droxy-11-[(E)-4-meth-oxy-benzyl-idene]-8-(4-meth-oxy-phen-yl)-5-thia-3,13-diaza-hepta-cyclo-[13.7.1.1.0.0.0.0]tetra-cosa-1(22),15(23),16,18,20-pentaen-10-one.Acta Crystallogr Sect E Struct Rep Online. 2011 Nov;67(Pt 11):o2881-2. doi: 10.1107/S160053681104061X. Epub 2011 Oct 8. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22219918 Free PMC article.
-
2-[6-(4-Bromo-phen-yl)imidazo[2,1-b][1,3]thia-zol-3-yl]-N-[8-(4-hy-droxy-phen-yl)-2-methyl-3-oxo-1-thia-4-aza-spiro-[4.5]decan-4-yl]acetamide ethanol disolvate.Acta Crystallogr Sect E Struct Rep Online. 2012 May 1;68(Pt 5):o1505-6. doi: 10.1107/S1600536812015371. Epub 2012 Apr 21. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22590376 Free PMC article.
Cited by
-
13-Benzyl-4,11-dihy-droxy-1,8-diphen-yl-2,9-di-thia-13-aza-dispiro-[4.1.4.3]tetra-decan-6-one.IUCrdata. 2021 Feb 26;6(Pt 2):x210210. doi: 10.1107/S2414314621002108. eCollection 2021 Feb. IUCrdata. 2021. PMID: 36338860 Free PMC article.
References
-
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. - PubMed
-
- Hema, R., Parthasarathi, V., Ravikumar, K., Sridhar, B. & Pandiarajan, K. (2005a). Acta Cryst. E61, o4345–o4347.
-
- Hema, R., Parthasarathi, V., Ravikumar, K., Sridhar, B., Pandiarajan, K. & Muthukumaran, G. (2005b). Acta Cryst. E61, o3987–o3989.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
