Crystal structure of N-[(1S,2S)-2-amino-cyclo-hex-yl]-2,4,6-tri-methyl-benzene-sulfonamide
- PMID: 26870419
- PMCID: PMC4719828
- DOI: 10.1107/S205698901502191X
Crystal structure of N-[(1S,2S)-2-amino-cyclo-hex-yl]-2,4,6-tri-methyl-benzene-sulfonamide
Abstract
The title compound, C15H24N2O2S, was synthesized via a substitution reaction between the enanti-opure (1S,2S)-(+)-1,2-di-amino-cyclo-hexane and 2,4,6-tri-methyl-benzene-1-sulfonyl chloride. The cyclo-hexyl and phenyl substituents are oriented gauche around the sulfonamide S-N bond. In the crystal, mol-ecules are linked via N-H⋯N hydrogen bonds, forming chains propagating along [100].
Keywords: chiral compound; crystal structure; hydrogen bond; sulfonamide.
Figures
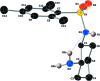
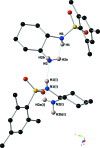
 , −y +
, −y +  , −z + 1.]
, −z + 1.]Similar articles
-
N,N'-[(1S,2S)-Cyclo-hexane-1,2-di-yl]bis-(4-methyl-benzene-sulfonamide).Acta Crystallogr Sect E Struct Rep Online. 2011 May 1;67(Pt 5):o1121. doi: 10.1107/S1600536811012372. Epub 2011 Apr 13. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21754435 Free PMC article.
-
Crystal structure of (E)-2-[(2S,5R)-2-isopropyl-5-methyl-cyclo-hexyl-idene]hydrazine-1-carbo-thio-amide.Acta Crystallogr Sect E Struct Rep Online. 2014 Aug 1;70(Pt 9):o903-4. doi: 10.1107/S1600536814015980. eCollection 2014 Sep 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 25309244 Free PMC article.
-
Crystal structures of (R S)-N-[(1R,2S)-2-benz-yloxy-1-(2,6-di-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide and (R S)-N-[(1S,2R)-2-benz-yloxy-1-(2,4,6-tri-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide: two related protected 1,2-amino alcohols.Acta Crystallogr Sect E Struct Rep Online. 2014 Oct 24;70(Pt 11):365-9. doi: 10.1107/S1600536814022570. eCollection 2014 Nov 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 25484747 Free PMC article.
-
Crystal structure of (1S,2R,4S)-1-[(morpholin-4-yl)meth-yl]-4-(prop-1-en-2-yl)cyclo-hexane-1,2-diol.Acta Crystallogr E Crystallogr Commun. 2015 Jan 1;71(Pt 1):79-81. doi: 10.1107/S2056989014027169. eCollection 2015 Jan 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25705457 Free PMC article.
-
N-[(1S,2S)-2-Amino-1,2-diphenyl-eth-yl]-4-methyl-benzene-sulfonamide [(S,S)-TsDPEN].Acta Crystallogr Sect E Struct Rep Online. 2010 Nov 27;66(Pt 12):o3343. doi: 10.1107/S1600536810049159. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21589615 Free PMC article.
Cited by
-
Crystal structure of phenyl 2,4,5-tri-chloro-benzene-sulfonate.Acta Crystallogr E Crystallogr Commun. 2016 May 6;72(Pt 6):789-92. doi: 10.1107/S2056989016007325. eCollection 2016 Jun 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 27308043 Free PMC article.
References
-
- Balsells, J., Mejorado, L., Phillips, M., Ortega, F., Aguirre, G., Somanathan, R. & Walsh, P. J. (1998). Tetrahedron Asymmetry, 9, 4135–4142.
-
- Bruker (2013). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2014). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
