Crystal structure of trans-di-aqua-bis-(1H-pyrazole-3-carboxyl-ato-κ(2) N,O)copper(II) dihydrate
- PMID: 26870440
- PMCID: PMC4719849
- DOI: 10.1107/S2056989015021593
Crystal structure of trans-di-aqua-bis-(1H-pyrazole-3-carboxyl-ato-κ(2) N,O)copper(II) dihydrate
Abstract
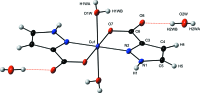
In the title compound, [Cu(C4H3N2O2)2(H2O)2]·2H2O, the Cu(II) ion is located on an inversion centre and exhibits an axially elongated octa-hedral coordination geometry. The equatorial plane is formed by two N,O-bidentate 1H-pyrazole-3-carboxyl-ate ligands in a trans configuration. The axial positions are occupied by two water mol-ecules. The mononuclear complex mol-ecules are arranged in layers parallel to the ab plane. Each complex mol-ecule is linked to four adjacent species through inter-molecular O-H⋯O and N-H⋯O hydrogen bonds that are established between the coordinating water mol-ecules and carboxyl-ate O atoms or protonated N atoms of the organic ligands. These layers are further connected into a three-dimensional network by additional hydrogen bonds involving solvent water mol-ecules and non-coordinating carboxyl-ate O atoms.
Keywords: 1H-pyrazole-3-carboxylate; copper(II) complex; crystal structure; trans configuration.
Figures

Similar articles
-
trans-Di-aqua-bis-(pyridazine-3-carboxyl-ato-κ(2) N (2),O)copper(II).Acta Crystallogr Sect E Struct Rep Online. 2014 Feb 28;70(Pt 3):m114-5. doi: 10.1107/S1600536814004334. eCollection 2014 Mar 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 24764943 Free PMC article.
-
trans-Di-aqua-bis-(pyridazine-3-carboxyl-ato-κ(2) N (2),O)cobalt(II) dihydrate.Acta Crystallogr Sect E Struct Rep Online. 2013 Jun 29;69(Pt 7):m420-1. doi: 10.1107/S1600536813017340. eCollection 2013. Acta Crystallogr Sect E Struct Rep Online. 2013. PMID: 24046586 Free PMC article.
-
Crystal structures of catena-poly[[μ-aqua-di-aqua(μ3-2-methyl-propano-ato-κ4 O:O,O':O')calcium] 2-methyl-propano-ate dihydrate], catena-poly[[μ-aqua-di-aqua-(μ3-2-methyl-propano-ato-κ4 O:O,O':O')strontium] 2-methyl-propano-ate dihydrate] and catena-poly[[μ-aqua-di-aqua-(μ3-2-methyl-propano-ato-κ4 O:O,O':O')(calcium/strontium)] 2-methyl-propano-ate dihydrate].Acta Crystallogr E Crystallogr Commun. 2020 Sep 25;76(Pt 10):1684-1688. doi: 10.1107/S2056989020012888. eCollection 2020 Oct 1. Acta Crystallogr E Crystallogr Commun. 2020. PMID: 33117590 Free PMC article.
-
Crystal structure of aqua-(1H-pyrazole-κN2)(pyridine-2,6-di-carboxyl-ato-κ3O2,N,O6)copper(II) dihydrate.Acta Crystallogr E Crystallogr Commun. 2017 Nov 17;73(Pt 12):1875-1877. doi: 10.1107/S2056989017016231. eCollection 2017 Dec 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29250406 Free PMC article.
-
Bis(3-amino-pyrazine-2-carboxyl-ato-κ(2) N (1),O)di-aqua-nickel(II) dihydrate.Acta Crystallogr Sect E Struct Rep Online. 2013 May 11;69(Pt 6):m309-10. doi: 10.1107/S1600536813012208. Print 2013 Jun 1. Acta Crystallogr Sect E Struct Rep Online. 2013. PMID: 23794977 Free PMC article.
Cited by
-
Structural characterization of two tetra-chlorido-zincate salts of 4-carb-oxy-1H-imidazol-3-ium: a salt hydrate and a co-crystal salt hydrate.Acta Crystallogr E Crystallogr Commun. 2017 Jan 13;73(Pt 2):162-167. doi: 10.1107/S2056989017000317. eCollection 2017 Feb 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28217334 Free PMC article.
References
-
- Agilent (2011). CrysAlis PRO. Agilent Technologies UK Ltd, Yarnton, England.
-
- Artetxe, B., Reinoso, S., San Felices, L., Vitoria, P., Pache, A., Martín-Caballero, J. & Gutiérrez-Zorrilla, J. M. (2015). Inorg. Chem. 54, 241–252. - PubMed
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- López-Viseras, M., Fernández, B., Hilfiker, S., González, C. S., González, J. L., Calahorro, A. J., Colacio, E. & Rodríguez-Diéguez, A. (2014). J. Inorg. Biochem. 131, 64–67. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
