Role of heparan sulfate in mediating CXCL8-induced endothelial cell migration
- PMID: 26870616
- PMCID: PMC4748698
- DOI: 10.7717/peerj.1669
Role of heparan sulfate in mediating CXCL8-induced endothelial cell migration
Abstract
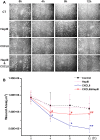
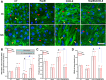
CXCL8 (Interleukin-8, IL-8) plays an important role in angiogenesis and wound healing by prompting endothelial cell migration. It has been suggested that heparan sulfate (HS) could provide binding sites on endothelial cells to retain and activate highly diffusible cytokines and inflammatory chemokines. In the present study, we aimed to test the hypothesis that HS is essential for enhancement of endothelial cell migration by CXCL8, and to explore the underlying mechanism by detecting the changes in expression and activity of Rho GTPases and in the organization of actin cytoskeleton after enzymatic removal of HS on human umbilical vein endothelial cells (HUVECs) by using heparinase III. Our results revealed that the wound healing induced by CXCL8 was greatly attenuated by removal of HS. The CXCL8-upregulated Rho GTPases including Cdc42, Rac1, and RhoA, and CXCL8-increased Rac1/Rho activity were suppressed by removal of HS. The polymerization and polarization of actin cytoskeleton, and the increasing of stress fibers induced by CXCL8 were also abolished by heparinase III. Taken together, our results demonstrated an essential role of HS in mediating CXCL8-induced endothelial cell migration, and highlighted the biological importance of the interaction between CXCL8 and heparan sulfate in wound healing.
Keywords: CXCL8; Cell migration; HUVEC; Heparan sulfate; Rho GTPases.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


Similar articles
-
Progesterone Inhibits Endothelial Cell Migration Through Suppression of the Rho Activity Mediated by cSrc Activation.J Cell Biochem. 2015 Jul;116(7):1411-8. doi: 10.1002/jcb.25101. J Cell Biochem. 2015. PMID: 25754581
-
Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases.J Cell Biol. 2003 Apr 28;161(2):429-39. doi: 10.1083/jcb.200210135. J Cell Biol. 2003. PMID: 12719476 Free PMC article.
-
A chemokine self-presentation mechanism involving formation of endothelial surface microstructures.J Immunol. 2013 Feb 15;190(4):1725-36. doi: 10.4049/jimmunol.1200867. Epub 2013 Jan 16. J Immunol. 2013. PMID: 23325889 Free PMC article.
-
Glycosaminoglycan-Mediated Downstream Signaling of CXCL8 Binding to Endothelial Cells.Int J Mol Sci. 2017 Dec 4;18(12):2605. doi: 10.3390/ijms18122605. Int J Mol Sci. 2017. PMID: 29207576 Free PMC article.
-
Role of GTPases in control of microvascular permeability.Cardiovasc Res. 2010 Jul 15;87(2):243-53. doi: 10.1093/cvr/cvq086. Epub 2010 Mar 17. Cardiovasc Res. 2010. PMID: 20299335 Review.
Cited by
-
Therapeutic Potential of Exosomes in Pulmonary Fibrosis.Front Pharmacol. 2020 Dec 4;11:590972. doi: 10.3389/fphar.2020.590972. eCollection 2020. Front Pharmacol. 2020. PMID: 33343360 Free PMC article. Review.
-
CXCL8 in Tumor Biology and Its Implications for Clinical Translation.Front Mol Biosci. 2022 Mar 15;9:723846. doi: 10.3389/fmolb.2022.723846. eCollection 2022. Front Mol Biosci. 2022. PMID: 35372515 Free PMC article. Review.
-
Fluid shear stress induces cell migration and invasion via activating autophagy in HepG2 cells.Cell Adh Migr. 2019 Dec;13(1):152-163. doi: 10.1080/19336918.2019.1568141. Epub 2019 Jan 31. Cell Adh Migr. 2019. PMID: 30663937 Free PMC article.
-
Plasma-Based Bioinks for Extrusion Bioprinting of Advanced Dressings.Biomedicines. 2021 Aug 16;9(8):1023. doi: 10.3390/biomedicines9081023. Biomedicines. 2021. PMID: 34440227 Free PMC article.
-
Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling.J Cell Mol Med. 2017 Aug;21(8):1457-1462. doi: 10.1111/jcmm.13081. Epub 2017 Feb 17. J Cell Mol Med. 2017. PMID: 28211170 Free PMC article. Review.
References
-
- Carveth HJ, Bohnsack JF, McIntyre TM, Baggiolini M, Prescott SM, Zimmerman GA. Neutrophil activating factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochemical and Biophysical Research Communications. 1989;162:387–393. doi: 10.1016/0006-291X(89)92009-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

