Mutations in the Mitochondrial Citrate Carrier SLC25A1 are Associated with Impaired Neuromuscular Transmission
- PMID: 26870663
- PMCID: PMC4746751
- DOI: 10.3233/JND-140021
Mutations in the Mitochondrial Citrate Carrier SLC25A1 are Associated with Impaired Neuromuscular Transmission
Abstract
Background and objective: Congenital myasthenic syndromes are rare inherited disorders characterized by fatigable weakness caused by malfunction of the neuromuscular junction. We performed whole exome sequencing to unravel the genetic aetiology in an English sib pair with clinical features suggestive of congenital myasthenia.
Methods: We used homozygosity mapping and whole exome sequencing to identify the candidate gene variants. Mutant protein expression and function were assessed in vitro and a knockdown zebrafish model was generated to assess neuromuscular junction development.
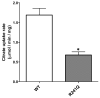
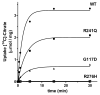
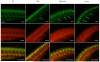
Results: We identified a novel homozygous missense mutation in the SLC25A1 gene, encoding the mitochondrial citrate carrier. Mutant SLC25A1 showed abnormal carrier function. SLC25A1 has recently been linked to a severe, often lethal clinical phenotype. Our patients had a milder phenotype presenting primarily as a neuromuscular (NMJ) junction defect. Of note, a previously reported patient with different compound heterozygous missense mutations of SLC25A1 has since been shown to suffer from a neuromuscular transmission defect. Using knockdown of SLC25A1 expression in zebrafish, we were able to mirror the human disease in terms of variable brain, eye and cardiac involvement. Importantly, we show clear abnormalities in the neuromuscular junction, regardless of the severity of the phenotype.
Conclusions: Based on the axonal outgrowth defects seen in SLC25A1 knockdown zebrafish, we hypothesize that the neuromuscular junction impairment may be related to pre-synaptic nerve terminal abnormalities. Our findings highlight the complex machinery required to ensure efficient neuromuscular function, beyond the proteomes exclusive to the neuromuscular synapse.
Keywords: Congenital myasthenic syndrome; SLC25A1; mitochondrial citrate carrier; neuromuscular junction.
Figures





Similar articles
-
Congenital myasthenic syndrome with mild intellectual disability caused by a recurrent SLC25A1 variant.Eur J Hum Genet. 2020 Mar;28(3):373-377. doi: 10.1038/s41431-019-0506-2. Epub 2019 Sep 16. Eur J Hum Genet. 2020. PMID: 31527857 Free PMC article.
-
Mitochondrial Mutations Can Alter Neuromuscular Transmission in Congenital Myasthenic Syndrome and Mitochondrial Disease.Int J Mol Sci. 2023 May 9;24(10):8505. doi: 10.3390/ijms24108505. Int J Mol Sci. 2023. PMID: 37239850 Free PMC article. Review.
-
[Congenital myasthenic syndrome related to SLC25A1 gene variant: two cases report and literature review].Zhonghua Er Ke Za Zhi. 2021 Jan 2;59(1):42-46. doi: 10.3760/cma.j.cn112140-20200730-00767. Zhonghua Er Ke Za Zhi. 2021. PMID: 33397003 Review. Chinese.
-
A case report of an intermediate phenotype between congenital myasthenic syndrome and D-2- and L-2-hydroxyglutaric aciduria due to novel SLC25A1 variants.BMC Neurol. 2020 Jul 13;20(1):278. doi: 10.1186/s12883-020-01854-6. BMC Neurol. 2020. PMID: 32660532 Free PMC article.
-
A novel homozygous SLC25A1 mutation with impaired mitochondrial complex V: Possible phenotypic expansion.Am J Med Genet A. 2018 Feb;176(2):330-336. doi: 10.1002/ajmg.a.38574. Epub 2017 Dec 11. Am J Med Genet A. 2018. PMID: 29226520
Cited by
-
Severe Neonatal Presentation of Mitochondrial Citrate Carrier (SLC25A1) Deficiency.JIMD Rep. 2016;30:73-79. doi: 10.1007/8904_2016_536. Epub 2016 Jun 16. JIMD Rep. 2016. PMID: 27306203 Free PMC article.
-
Clinical and Pathologic Features of Congenital Myasthenic Syndromes Caused by 35 Genes-A Comprehensive Review.Int J Mol Sci. 2023 Feb 13;24(4):3730. doi: 10.3390/ijms24043730. Int J Mol Sci. 2023. PMID: 36835142 Free PMC article. Review.
-
APOE expression and secretion are modulated by mitochondrial dysfunction.Elife. 2023 May 12;12:e85779. doi: 10.7554/eLife.85779. Elife. 2023. PMID: 37171075 Free PMC article.
-
The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond.Biomolecules. 2021 Jan 22;11(2):141. doi: 10.3390/biom11020141. Biomolecules. 2021. PMID: 33499062 Free PMC article. Review.
-
[Advances in the diagnosis and treatment of congenital myasthenic syndrome].Zhongguo Dang Dai Er Ke Za Zhi. 2020 Jun;22(6):672-676. doi: 10.7499/j.issn.1008-8830.1912095. Zhongguo Dang Dai Er Ke Za Zhi. 2020. PMID: 32571471 Free PMC article. Chinese.
References
-
- Beeson D. Synaptic dysfunction in congenital myasthenic syndromes. Annals of the New York Academy of Sciences. 2012;1275:63–69. - PubMed
-
- Chaouch A, Beeson D, Hantai D, Lochmuller H. 186th ENMC international workshop: Congenital myasthenic syndromes 24-26 June 2011, Naarden, The Netherlands. Neuromuscular Disorders: NMD. 2012;22(6):566–576. - PubMed
-
- Barisic N, Chaouch A, Muller JS, Lochmuller H. Genetic heterogeneity and pathophysiological mechanisms in congenital myasthenic syndromes. Eur J Paediatr Neurol. 2011;15(3):189–196. - PubMed
-
- Hantai D, Nicole S, Eymard B. Congenital myasthenic syndromes: An update. Current Opinion in Neurology. 2013;26(5):561–568. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

