Infected cell protein 0 functional domains and their coordination in herpes simplex virus replication
- PMID: 26870669
- PMCID: PMC4735549
- DOI: 10.5501/wjv.v5.i1.1
Infected cell protein 0 functional domains and their coordination in herpes simplex virus replication
Abstract
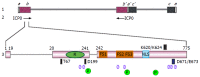
Herpes simplex virus 1 (HSV-1) is a ubiquitous human pathogen that establishes latent infection in ganglia neurons. Its unique life cycle requires a balanced "conquer and compromise" strategy to deal with the host anti-viral defenses. One of HSV-1 α (immediate early) gene products, infected cell protein 0 (ICP0), is a multifunctional protein that interacts with and modulates a wide range of cellular defensive pathways. These pathways may locate in different cell compartments, which then migrate or exchange factors upon stimulation, for the purpose of a concerted and effective defense. ICP0 is able to simultaneously attack multiple host pathways by either degrading key restrictive factors or modifying repressive complexes. This is a viral protein that contains an E3 ubiquitin ligase, translocates among different cell compartments and interacts with major defensive complexes. The multiple functional domains of ICP0 can work independently and at the same time coordinate with each other. Dissecting the functional domains of ICP0 and delineating the coordination of these domains will help us understand HSV-1 pathogenicity as well as host defense mechanisms. This article focuses on describing individual ICP0 domains, their biochemical properties and their implication in HSV-1 infection. By putting individual domain functions back into the picture of host anti-viral defense network, this review seeks to elaborate the complex interactions between HSV-1 and its host.
Keywords: Chromatin repression; E3 ubiquitin ligase; Herpes simplex virus 1; Infected cell protein 0; ND10 nuclear bodies; Protein modification; Subcellular translocation.
Figures
Similar articles
-
A Tale of Two PMLs: Elements Regulating a Differential Substrate Recognition by the ICP0 E3 Ubiquitin Ligase of Herpes Simplex Virus 1.J Virol. 2016 Nov 14;90(23):10875-10885. doi: 10.1128/JVI.01636-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681131 Free PMC article.
-
Role of ND10 nuclear bodies in the chromatin repression of HSV-1.Virol J. 2016 Apr 5;13:62. doi: 10.1186/s12985-016-0516-4. Virol J. 2016. PMID: 27048561 Free PMC article. Review.
-
Characterization of Elements Regulating the Nuclear-to-Cytoplasmic Translocation of ICP0 in Late Herpes Simplex Virus 1 Infection.J Virol. 2018 Jan 2;92(2):e01673-17. doi: 10.1128/JVI.01673-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093084 Free PMC article.
-
Identification of three redundant segments responsible for herpes simplex virus 1 ICP0 to fuse with ND10 nuclear bodies.J Virol. 2015 Apr;89(8):4214-26. doi: 10.1128/JVI.03658-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631093 Free PMC article.
-
Discovery of Small-Molecule Inhibitors Targeting the E3 Ubiquitin Ligase Activity of the Herpes Simplex Virus 1 ICP0 Protein Using an In Vitro High-Throughput Screening Assay.J Virol. 2019 Jun 14;93(13):e00619-19. doi: 10.1128/JVI.00619-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996104 Free PMC article.
Cited by
-
A Tale of Two PMLs: Elements Regulating a Differential Substrate Recognition by the ICP0 E3 Ubiquitin Ligase of Herpes Simplex Virus 1.J Virol. 2016 Nov 14;90(23):10875-10885. doi: 10.1128/JVI.01636-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681131 Free PMC article.
-
Herpes simplex virus and the lexicon of latency and reactivation: a call for defining terms and building an integrated collective framework.F1000Res. 2016 Aug 19;5:F1000 Faculty Rev-2038. doi: 10.12688/f1000research.8886.1. eCollection 2016. F1000Res. 2016. PMID: 27610228 Free PMC article. Review.
-
In Vitro Effect of 9,9'-Norharmane Dimer against Herpes Simplex Viruses.Int J Mol Sci. 2024 May 2;25(9):4966. doi: 10.3390/ijms25094966. Int J Mol Sci. 2024. PMID: 38732185 Free PMC article.
-
Herpes simplex virus type I-infected disorders alter the balance between Treg and Th17 cells in recurrent herpes labialis patients.Int J Immunopathol Pharmacol. 2020 Jan-Dec;34:2058738420933099. doi: 10.1177/2058738420933099. Int J Immunopathol Pharmacol. 2020. PMID: 32735468 Free PMC article.
-
Interactome and Ubiquitinome Analyses Identify Functional Targets of Herpes Simplex Virus 1 Infected Cell Protein 0.Front Microbiol. 2022 Apr 18;13:856471. doi: 10.3389/fmicb.2022.856471. eCollection 2022. Front Microbiol. 2022. PMID: 35516420 Free PMC article.
References
-
- Roizman B, Knipe DM. Whitley RJ. Herpes Simplex Viruses, in Fields Virology, 6 Edition, Lippincott Williams & Wilkins. 2013. pp. 1823–1897.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

