BET Bromodomain Blockade Mitigates Intimal Hyperplasia in Rat Carotid Arteries
- PMID: 26870791
- PMCID: PMC4740308
- DOI: 10.1016/j.ebiom.2015.09.045
BET Bromodomain Blockade Mitigates Intimal Hyperplasia in Rat Carotid Arteries
Abstract
Background: Intimal hyperplasia is a common cause of many vasculopathies. There has been a recent surge of interest in the bromo and extra-terminal (BET) epigenetic "readers" including BRD4 since the serendipitous discovery of JQ1(+), an inhibitor specific to the seemingly undruggable BET bromodomains. The role of the BET family in the development of intimal hyperplasia is not known.
Methods: We investigated the effect of BET inhibition on intimal hyperplasia using a rat balloon angioplasty model.
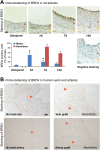
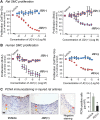
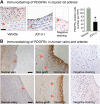
Results: While BRD4 was dramatically up-regulated in the rat and human hyperplastic neointima, blocking BET bromodomains with JQ1(+) diminished neointima in rats. Knocking down BRD4 with siRNA, or treatment with JQ1(+) but not the inactive enantiomer JQ1(-), abrogated platelet-derived growth factor (PDGF-BB)-stimulated proliferation and migration of primary rat aortic smooth muscle cells. This inhibitory effect of JQ1(+) was reproducible in primary human aortic smooth muscle cells. In human aortic endothelial cells, JQ1(+) prevented cytokine-induced apoptosis and impairment of cell migration. Furthermore, either BRD4 siRNA or JQ1(+) but not JQ1(-), substantially down-regulated PDGF receptor-α which, in JQ1(+)-treated arteries versus vehicle control, was also reduced.
Conclusions: Blocking BET bromodomains mitigates neointima formation, suggesting an epigenetic approach for effective prevention of intimal hyperplasia and associated vascular diseases.
Keywords: BET; BET, bromo- and extra-terminal domain family of epigenetic readers; BRD4; BRD4, bromodomain-containing protein 4, a BET family member; EC, vascular endothelial cells; Epigenetic reader; IH, intimal hyperplasia; Intimal hyperplasia; JQ1(+), a BET-specific bromodomain inhibitor; JQ1(−), inactive enantiomer; PDGF, platelet-derived growth factor; SMC, vascular smooth muscle cell; Smooth muscle cell.
Figures





Comment in
-
Bromodomain Blockade for Intimal Hyperplasia--A Good BET?EBioMedicine. 2015 Nov 10;2(11):1574-5. doi: 10.1016/j.ebiom.2015.11.007. eCollection 2015 Nov. EBioMedicine. 2015. PMID: 26870770 Free PMC article. No abstract available.
Similar articles
-
BET bromodomain-containing epigenetic reader proteins regulate vascular smooth muscle cell proliferation and neointima formation.Cardiovasc Res. 2021 Feb 22;117(3):850-862. doi: 10.1093/cvr/cvaa121. Cardiovasc Res. 2021. PMID: 32353113
-
PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells.Cardiovasc Res. 2011 Dec 1;92(3):484-93. doi: 10.1093/cvr/cvr238. Epub 2011 Aug 31. Cardiovasc Res. 2011. PMID: 21880694
-
Hydrogen-rich saline prevents neointima formation after carotid balloon injury by suppressing ROS and the TNF-α/NF-κB pathway.Atherosclerosis. 2012 Feb;220(2):343-50. doi: 10.1016/j.atherosclerosis.2011.11.002. Epub 2011 Nov 11. Atherosclerosis. 2012. PMID: 22153150
-
BET bromodomain inhibitors--a novel epigenetic approach in castration-resistant prostate cancer.Cancer Biol Ther. 2014;15(12):1583-5. doi: 10.4161/15384047.2014.962297. Cancer Biol Ther. 2014. PMID: 25535892 Free PMC article. Review.
-
Bromodomains: Structure, function and pharmacology of inhibition.Biochem Pharmacol. 2016 Apr 15;106:1-18. doi: 10.1016/j.bcp.2015.12.005. Epub 2015 Dec 18. Biochem Pharmacol. 2016. PMID: 26707800 Review.
Cited by
-
Angioplasty induces epigenomic remodeling in injured arteries.Life Sci Alliance. 2022 Feb 15;5(5):e202101114. doi: 10.26508/lsa.202101114. Print 2022 May. Life Sci Alliance. 2022. PMID: 35169042 Free PMC article.
-
Computational repurposing of therapeutic small molecules from cancer to pulmonary hypertension.Sci Adv. 2021 Oct 22;7(43):eabh3794. doi: 10.1126/sciadv.abh3794. Epub 2021 Oct 20. Sci Adv. 2021. PMID: 34669463 Free PMC article.
-
Epigenetics and vascular diseases.J Mol Cell Cardiol. 2019 Aug;133:148-163. doi: 10.1016/j.yjmcc.2019.06.010. Epub 2019 Jun 15. J Mol Cell Cardiol. 2019. PMID: 31211956 Free PMC article. Review.
-
Ursodeoxycholic acid inhibits intimal hyperplasia, vascular smooth muscle cell excessive proliferation, migration via blocking miR-21/PTEN/AKT/mTOR signaling pathway.Cell Cycle. 2020 Apr;19(8):918-932. doi: 10.1080/15384101.2020.1732514. Epub 2020 Mar 22. Cell Cycle. 2020. Update in: Cell Cycle. 2022 Apr;21(8):881. doi: 10.1080/15384101.2022.2046824. PMID: 32202193 Free PMC article. Updated.
-
Targeting smooth muscle cell phenotypic switching in vascular disease.JVS Vasc Sci. 2021 May 15;2:79-94. doi: 10.1016/j.jvssci.2021.04.001. eCollection 2021. JVS Vasc Sci. 2021. PMID: 34617061 Free PMC article. Review.
References
-
- Alexander M.R., Owens G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012;74:13–40. - PubMed
-
- Amaral P.P., Bannister A.J. Re-place your BETs: the dynamics of super enhancers. Mol. Cell. 2014;56:187–189. - PubMed
-
- Asangani I.A., Dommeti V.L., Wang X., Malik R., Cieslik M., Yang R., Escara-Wilke J., Wilder-Romans K., Dhanireddy S., Engelke C., Iyer M.K., Jing X., Wu Y.M., Cao X., Qin Z.S., Wang S., Feng F.Y., Chinnaiyan A.M. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

