The Sigma-2 Receptor and Progesterone Receptor Membrane Component 1 are Different Binding Sites Derived From Independent Genes
- PMID: 26870805
- PMCID: PMC4740303
- DOI: 10.1016/j.ebiom.2015.10.017
The Sigma-2 Receptor and Progesterone Receptor Membrane Component 1 are Different Binding Sites Derived From Independent Genes
Abstract
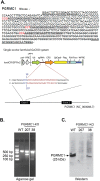
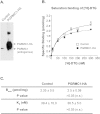
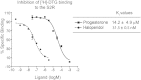
The sigma-2 receptor (S2R) is a potential therapeutic target for cancer and neuronal diseases. However, the identity of the S2R has remained a matter of debate. Historically, the S2R has been defined as (1) a binding site with high affinity to 1,3-di-o-tolylguanidine (DTG) and haloperidol but not to the selective sigma-1 receptor ligand (+)-pentazocine, and (2) a protein of 18-21 kDa, as shown by specific photolabeling with [(3)H]-Azido-DTG and [(125)I]-iodoazido-fenpropimorph ([(125)I]-IAF). Recently, the progesterone receptor membrane component 1 (PGRMC1), a 25 kDa protein, was reported to be the S2R (Nature Communications, 2011, 2:380). To confirm this identification, we created PGRMC1 knockout NSC34 cell lines using the CRISPR/Cas9 technology. We found that in NSC34 cells devoid of or overexpressing PGRMC1, the maximum [(3)H]-DTG binding to the S2R (Bmax) as well as the DTG-protectable [(125)I]-IAF photolabeling of the S2R were similar to those of wild-type control cells. Furthermore, the affinities of DTG and haloperidol for PGRMC1 (KI = 472 μM and 350 μM, respectively), as determined in competition with [(3)H]-progesterone, were more than 3 orders of magnitude lower than those reported for the S2R (20-80 nM). These results clarify that PGRMC1 and the S2R are distinct binding sites expressed by different genes.
Keywords: Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 knockout; Progesterone receptor membrane component-1 (PGRMC1); Sigma-2 receptor (S2R); [125I]-Iodoazido-fenpropimorph ([125I]-IAF); [3H]-1,3-Di-o-tolylguanidine ([3H]-DTG).
Figures



References
-
- Abate C., Niso M., Infantino V., Menga A., Berardi F. Elements in support of the 'non-identity' of the PGRMC1 protein with the sigma2 receptor. Eur. J. Pharmacol. 2015;758:16–23. - PubMed
-
- Bowen W.D., Hellewell S.B., Mcgarry K.A. Evidence for a multi-site model of the rat brain sigma receptor. Eur. J. Pharmacol. 1989;163:309–318. - PubMed
-
- Cassano G., Gasparre G., Contino M., Niso M., Berardi F., Perrone R., Colabufo N.A. The sigma-2 receptor agonist PB28 inhibits calcium release from the endoplasmic reticulum of SK-N-SH neuroblastoma cells. Cell Calcium. 2006;40:23–28. - PubMed
-
- Chu U.B., M.T A., Chu M.-L., Yang H., Mesangeau C., McCurdy C.R., Guo L.-W., Ruoho A.E. Experimental Biology Meeting. 2015. The 18 kDa sigma-2 receptor and PGRMC1 are derived from separate genes. (LB511)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

