Role of reactive oxygen species in age-related neuromuscular deficits
- PMID: 26870901
- PMCID: PMC4933106
- DOI: 10.1113/JP270564
Role of reactive oxygen species in age-related neuromuscular deficits
Abstract
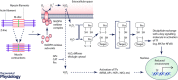
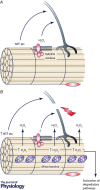
Although it is now clear that reactive oxygen species (ROS) are not the key determinants of longevity, a number of studies have highlighted the key role that these species play in age-related diseases and more generally in determining individual health span. Age-related loss of skeletal muscle mass and function is a key contributor to physical frailty in older individuals and our current understanding of the key areas in which ROS contribute to age-related deficits in muscle is through defective redox signalling and key roles in maintenance of neuromuscular integrity. This topical review will describe how ROS stimulate adaptations to contractile activity in muscle that include up-regulation of short-term stress responses, an increase in mitochondrial biogenesis and an increase in some catabolic processes. These adaptations occur through stimulation of redox-regulated processes that lead to the activation of transcription factors such as NF-κB, AP-1 and HSF1 which mediate changes in gene expression. They are attenuated during ageing and this appears to occur through an age-related increase in mitochondrial hydrogen peroxide production. The potential for redox-mediated cross-talk between motor neurons and muscle is also described to illustrate how ROS released from muscle fibres during exercise may help maintain the integrity of axons and how the degenerative changes in neuromuscular structure that occur with ageing may contribute to mitochondrial ROS generation in skeletal muscle fibres.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
References
-
- Adinolfi AM, Yamuy J, Morales FR & Chase MH (1991). Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging 12, 175–179. - PubMed
-
- Aggeli IK, Gaitanaki C & Beis I (2006). Involvement of JNKs and p38‐MAPK/MSK1 pathways in H2O2‐induced upregulation of heme oxygenase‐1 mRNA in H9c2 cells. Cell Signal 18, 1801–1812. - PubMed
-
- Bhattacharya A, Hamilton R, Jernigan A, Zhang Y, Sabia M, Rahman MM, Li Y, Wei R, Chaudhuri A & Van Remmen H (2014). Genetic ablation of 12/15‐lipoxygenase but not 5‐lipoxygenase protects against denervation‐induced muscle atrophy. Free Radic Biol Med 67, 30–40. - PubMed
-
- Bishop A, Marquis JC, Cashman NR & Demple B (1999). Adaptive resistance to nitric oxide in motor neurons. Free Radic Biol Med 26, 978–986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

