Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer
- PMID: 26871285
- PMCID: PMC4914299
- DOI: 10.18632/oncotarget.7245
Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer
Abstract
Prostate-specific membrane antigen (PSMA) is highly expressed on prostate epithelial cells and strongly up-regulated in prostate cancer (PC), making it an optimal target for the treatment of metastasized PC. Radioligand therapy (RLT) with 177Lu-PSMA-DKFZ-617 (Lu-PSMA) is a targeted therapy for metastatic PC. In this study, we retrospectively analyzed the side effects and the response rate of 24 hormone and/or chemorefractory PC patients with a mean age of 75.2 years (range: 64-82) with distant metastases and progressive disease according to the PSA level, who were treated with Lu-PSMA. Median PSA was 522 ng/ml (range: 17-2360). Forty-six cycles of Lu-PSMA were performed. Of the 24 patients, 22 received two cycles. Eight weeks after the first cycle of Lu-PSMA therapy 79.1% experienced a decline in PSA level. Eight weeks after the second cycle of Lu-PSMA therapy 68.2% experienced a decline in PSA relative to the baseline value. Apart from two cases of grade 3 anemia, there was no relevant hemato- or nephrotoxicity (grade 3 or 4). These results confirmed that Lu-PSMA is a safe treatment option for metastatic PC patients and has a low toxicity profile. A positive response to therapy in terms of decline in PSA occurs in about 70% of patients.
Keywords: 177Lu; PSA; PSMA; prostate cancer; radioligand therapy.
Conflict of interest statement
The authors declare that they have no financial and non-financial competing interests.
Figures


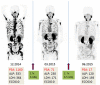
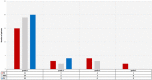


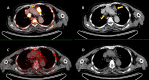
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–386. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. - PubMed
-
- Wei Q, Li M, Fu X, Tang R, Na Y, Jiang M, Li Y. Global analysis of differentially expressed genes in androgen-independent prostate cancer. Prostate cancer and prostatic diseases. 2007;10:167–174. - PubMed
-
- Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, Montironi R. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. Journal of biological regulators and homeostatic agents. 2014;28:555–563. - PubMed
-
- Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benesova M, Mier W, Kopka K, Haberkorn U. [Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. European journal of nuclear medicine and molecular imaging. 2015;42:987–988. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

