Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents
- PMID: 26871476
- PMCID: PMC4924706
- DOI: 10.18632/oncotarget.7290
Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents
Abstract
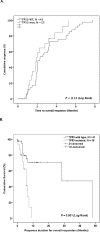
We screened TP53 mutations in 168 MDS patients who were treated with HMA and evaluated predictive and prognostic value of TP53 mutations. Overall response to HMA was not different based on TP53 mutation status (45% vs. 32% in TP53-mutated and wild type [WT], respectively, P = 0.13). However, response duration was significantly shorter in TP53-mutated patients compared to WT patients (5.7 months vs. 28.5 months, P = 0.003). Longitudinal analysis of TP53 mutations after HMA showed that TP53 mutations almost always persisted at times of disease progression. TP53-mutated patients showed significantly worse overall survival (OS) compared to WT patients (9.4 months vs. 20.7 months, P <0.001). Further, TP53 mutations distinguished prognosis in the subgroup of patients with complex karyotype and Revised International Prognostic Scoring System (IPSS-R) defined very high-risk disease. Multivariate analysis showed that TP53 mutation status is significantly prognostic for OS after adjusting prognostic effect from other factors. The current study provides evidence that TP53 mutations are independently prognostic in MDS patients treated with HMA. While TP53-mutated MDS patients initially respond well to HMA, their duration of response is significantly shorter than WT patients. Novel strategies to improve duration of response in TP53-mutated MDS are urgently needed.
Keywords: TP53; hypomethylating agents; myelodysplastic syndromes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 2007;26:2145–2156. - PubMed
-
- Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer cell. 2007;12:303–312. - PubMed
-
- Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, Wang H, Caughey B, Stojanov P, Getz G, Garcia-Manero G, Kantarjian H, Chen R, Stone RM, Neuberg D, Steensma DP, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705–2712. - PMC - PubMed
-
- Kulasekararaj AG, Smith AE, Mian SA, Mohamedali AM, Krishnamurthy P, Lea NC, Gaken J, Pennaneach C, Ireland R, Czepulkowski B, Pomplun S, Marsh JC, Mufti GJ. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. British journal of haematology. 2013;160:660–672. - PubMed
-
- Jadersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Gohring G, Hedlund A, Hast R, Schlegelberger B, Porwit A, Hellstrom-Lindberg E, Mufti GJ. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. Journal of clinical oncology. 2011;29:1971–1979. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

