Cisplatin-Induced Non-Oliguric Acute Kidney Injury in a Pediatric Experimental Animal Model in Piglets
- PMID: 26871589
- PMCID: PMC4752347
- DOI: 10.1371/journal.pone.0149013
Cisplatin-Induced Non-Oliguric Acute Kidney Injury in a Pediatric Experimental Animal Model in Piglets
Erratum in
-
Correction: Cisplatin-Induced Non-Oliguric Acute Kidney Injury in a Pediatric Experimental Animal Model in Piglets.PLoS One. 2018 Nov 9;13(11):e0207547. doi: 10.1371/journal.pone.0207547. eCollection 2018. PLoS One. 2018. PMID: 30412623 Free PMC article.
Abstract
Objective: To design an experimental pediatric animal model of acute kidney injury induced by cisplatin.
Methods: Prospective comparative observational animal study in two different phases. Acute kidney injury was induced using three different doses of cisplatin (2, 3 and 5 mg/kg). The development of nephrotoxicity was assessed 2 to 4 days after cisplatin administration by estimating biochemical parameters, diuresis and renal morphology. Analytical values and renal morphology were compared between 15 piglets treated with cisplatin 3 mg/kg and 15 control piglets in the second phase of the study.
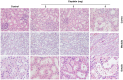
Results: 41 piglets were studied. The dose of 3 mg/kg administered 48 hours before the experience induced a significant increase in serum creatinine and urea without an increase in potassium levels. Piglets treated with cisplatin 3 mg/kg had significantly higher values of creatinine, urea, phosphate and amylase, less diuresis and lower values of potassium, sodium and bicarbonate than control piglets. Histological findings showed evidence of a dose-dependent increase in renal damage.
Conclusions: a dose of 3 mg/kg of cisplatin induces a significant alteration in renal function 48 hours after its administration, so it can be used as a pediatric animal model of non-oliguric acute kidney injury.
Conflict of interest statement
Figures
References
-
- Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med 12:339–47, 2011. - PubMed
-
- Stein JH, Fried TA. Experimental models of nephrotoxic acute renal failure. Transplant Proc 17(4 Suppl 1):72.–, 1985. - PubMed
-
- Lieberthal W, Nigam SK. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable Am J Physiol Renal Physiol 278: F1–F12, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources