Myc Depletion Induces a Pluripotent Dormant State Mimicking Diapause
- PMID: 26871632
- PMCID: PMC4752822
- DOI: 10.1016/j.cell.2015.12.033
Myc Depletion Induces a Pluripotent Dormant State Mimicking Diapause
Abstract
Mouse embryonic stem cells (ESCs) are maintained in a naive ground state of pluripotency in the presence of MEK and GSK3 inhibitors. Here, we show that ground-state ESCs express low Myc levels. Deletion of both c-myc and N-myc (dKO) or pharmacological inhibition of Myc activity strongly decreases transcription, splicing, and protein synthesis, leading to proliferation arrest. This process is reversible and occurs without affecting pluripotency, suggesting that Myc-depleted stem cells enter a state of dormancy similar to embryonic diapause. Indeed, c-Myc is depleted in diapaused blastocysts, and the differential expression signatures of dKO ESCs and diapaused epiblasts are remarkably similar. Following Myc inhibition, pre-implantation blastocysts enter biosynthetic dormancy but can progress through their normal developmental program after transfer into pseudo-pregnant recipients. Our study shows that Myc controls the biosynthetic machinery of stem cells without affecting their potency, thus regulating their entry and exit from the dormant state.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



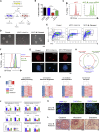

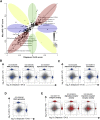
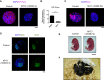
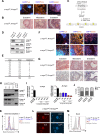


Comment in
-
Pluripotency without Proliferation.Cell. 2016 Feb 11;164(4):595-7. doi: 10.1016/j.cell.2016.01.050. Cell. 2016. PMID: 26871623
References
-
- Adhikary S., Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005;6:635–645. - PubMed
-
- Arnold I., Watt F.M. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 2001;11:558–568. - PubMed
-
- Batlle-Morera L., Smith A., Nichols J. Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis. 2008;46:758–767. - PubMed
-
- Blackwood E.M., Eisenman R.N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

