Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor
- PMID: 26871944
- PMCID: PMC4924668
- DOI: 10.18632/oncotarget.7296
Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor
Abstract
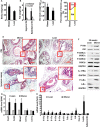
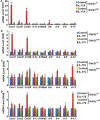
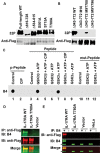
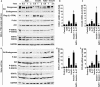
Interleukin-17 (IL-17) plays important roles in inflammation, autoimmune diseases, and some cancers. Obese people are in a chronic inflammatory state with increased serum levels of IL-17, insulin, and insulin-like growth factor 1 (IGF1). How these factors contribute to the chronic inflammatory status that promotes development of aggressive prostate cancer in obese men is largely unknown. We found that, in obese mice, hyperinsulinemia enhanced IL-17-induced expression of downstream proinflammatory genes with increased levels of IL-17 receptor A (IL-17RA), resulting in development of more invasive prostate cancer. Glycogen synthase kinase 3 (GSK3) constitutively bound to and phosphorylated IL-17RA at T780, leading to ubiquitination and proteasome-mediated degradation of IL-17RA, thus inhibiting IL-17-mediated inflammation. IL-17RA phosphorylation was reduced, while the IL-17RA levels were increased in the proliferative human prostate cancer cells compared to the normal cells. Insulin and IGF1 enhanced IL-17-induced inflammatory responses through suppressing GSK3, which was shown in the cultured cell lines in vitro and obese mouse models of prostate cancer in vivo. These findings reveal a mechanism underlying the intensified inflammation in obesity and obesity-associated development of aggressive prostate cancer, suggesting that targeting GSK3 may be a potential therapeutic approach to suppress IL-17-mediated inflammation in the prevention and treatment of prostate cancer, particularly in obese men.
Keywords: GSK3; hyperinsulinemia; interleukin-17; obesity; prostate cancer.
Conflict of interest statement
The authors declared no conflicts of interests.
Figures


References
-
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. - PubMed
-
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, Olsen H, Ruben SM, Knyazev I, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

