The sensitivity and significance of lateralized interictal slow activity on magnetoencephalography in focal epilepsy
- PMID: 26871959
- PMCID: PMC4769925
- DOI: 10.1016/j.eplepsyres.2016.01.009
The sensitivity and significance of lateralized interictal slow activity on magnetoencephalography in focal epilepsy
Abstract
Objective: Asymmetric large-amplitude slow activity is sometimes observed on interictal electroencephalography (EEG) in epilepsy. However, few studies have examined slowing during magnetoencephalography (MEG) recordings, which are performed primarily to localize interictal spikes. Also, no prior investigations have compared the sensitivity of MEG to scalp EEG in detecting slow rhythms.
Methods: We performed a retrospective cohort study of focal epilepsy patients who received MEG followed by surgical resection at our institution. We examined MEG, simultaneous EEG, and long-term EEG recordings for prominent asymmetric slow activity (delta-range, 1-4 Hz), and evaluated post-operative seizure outcomes.
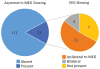
Results: We studied 132 patients with ≥ 1 year post-operative follow-up (mean, 3.6 years). Mean age was 27 (range, 3-68) years, and 55% of patients were male. Asymmetric large-amplitude slow wave activity was observed on interictal MEG in 21 of 132 (16%) patients. Interictal slowing lateralized to the hemisphere of resection in all but one (95%) patient. Among the 21 patients with interictal MEG slowing, 11 (52%) individuals had similarly lateralized EEG slowing, 7 patients had no EEG slowing, and 3 had bilateral symmetric EEG slowing. Meanwhile, none of the 111 patients without lateralized MEG slowing had asymmetric EEG slowing, suggesting significantly higher sensitivity of MEG versus EEG in detecting asymmetric slowing (χ(2)=63.4, p<0.001). MEG slowing was associated with shorter epilepsy duration with an odds ratio of 5.4 (1.7-17.0, 95% confidence interval). At last follow-up, 92 (70%) patients were seizure free (Engel I outcome), with no difference in seizure freedom rates between patients with (71%) or without (69%) asymmetric MEG slowing (χ(2)=0.4, p=0.99).
Significance: MEG has higher sensitivity than scalp EEG in detecting asymmetric slow activity in focal epilepsy, which reliably lateralizes to the epileptogenic hemisphere. Other uses of MEG beyond spike localization may further improve presurgical evaluations in epilepsy.
Keywords: Epilepsy surgery; Epileptogenic zone; MEG; Magnetic source imaging; Slowing.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
disclosures: The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery.Epilepsia. 2015 Jun;56(6):949-58. doi: 10.1111/epi.13002. Epub 2015 Apr 29. Epilepsia. 2015. PMID: 25921215 Free PMC article.
-
Detection and significance of focal, interictal, slow-wave activity visualized by magnetoencephalography for localization of a primary epileptogenic region.J Neurosurg. 2002 Apr;96(4):724-30. doi: 10.3171/jns.2002.96.4.0724. J Neurosurg. 2002. PMID: 11990813
-
Accuracy of MEG in localizing irritative zone and seizure onset zone: Quantitative comparison between MEG and intracranial EEG.Epilepsy Res. 2016 Nov;127:291-301. doi: 10.1016/j.eplepsyres.2016.08.013. Epub 2016 Aug 16. Epilepsy Res. 2016. PMID: 27693985
-
Magnetoencephalography in focal epilepsy.Epilepsia. 2000;41 Suppl 3:S39-47. doi: 10.1111/j.1528-1157.2000.tb01533.x. Epilepsia. 2000. PMID: 11001335 Review.
-
Controversies in clinical neurophysiology. MEG is superior to EEG in the localization of interictal epileptiform activity: Con.Clin Neurophysiol. 2004 May;115(5):1010-20. doi: 10.1016/j.clinph.2003.12.010. Clin Neurophysiol. 2004. PMID: 15066524 Review.
Cited by
-
Interictal network dysfunction and cognitive impairment in epilepsy.Nat Rev Neurosci. 2025 Jul;26(7):399-414. doi: 10.1038/s41583-025-00924-3. Epub 2025 Apr 28. Nat Rev Neurosci. 2025. PMID: 40295879 Review.
-
Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings.Epilepsia. 2016 Oct;57(10):1546-1557. doi: 10.1111/epi.13510. Epub 2016 Aug 24. Epilepsia. 2016. PMID: 27554793 Free PMC article. Review.
-
Lateralization of delta band power in magnetoencephalography (MEG) in patients with unilateral focal epilepsy and its relation to verbal fluency.Brain Behav. 2023 Nov;13(11):e3257. doi: 10.1002/brb3.3257. Epub 2023 Sep 26. Brain Behav. 2023. PMID: 37752097 Free PMC article.
-
Multi-Head Self-Attention Model for Classification of Temporal Lobe Epilepsy Subtypes.Front Physiol. 2020 Nov 27;11:604764. doi: 10.3389/fphys.2020.604764. eCollection 2020. Front Physiol. 2020. PMID: 33329057 Free PMC article.
-
Localization of the Epileptogenic Zone Using Interictal MEG and Machine Learning in a Large Cohort of Drug-Resistant Epilepsy Patients.Front Neurol. 2018 Aug 7;9:647. doi: 10.3389/fneur.2018.00647. eCollection 2018. Front Neurol. 2018. PMID: 30131762 Free PMC article.
References
-
- Baayen JC, de Jongh A, Stam CJ, de Munck JC, Jonkman JJ, Trenite DG, Berendse HW, van Walsum AM, Heimans JJ, Puligheddu M, Castelijns JA, Vandertop WP. Localization of slow wave activity in patients with tumor-associated epilepsy. Brain topography. 2003;16:85–93. - PubMed
-
- Blume WT, Borghesi JL, Lemieux JF. Interictal indices of temporal seizure origin. Annals of neurology. 1993;34:703–709. - PubMed
-
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004a;14:892–902. - PubMed
-
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004b;63:1015–1021. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

