Specific allergen immunotherapy for the treatment of atopic eczema
- PMID: 26871981
- PMCID: PMC8761476
- DOI: 10.1002/14651858.CD008774.pub2
Specific allergen immunotherapy for the treatment of atopic eczema
Abstract
Background: Specific allergen immunotherapy (SIT) is a treatment that may improve disease severity in people with atopic eczema (AE) by inducing immune tolerance to the relevant allergen. A high quality systematic review has not previously assessed the efficacy and safety of this treatment.
Objectives: To assess the effects of specific allergen immunotherapy (SIT), including subcutaneous, sublingual, intradermal, and oral routes, compared with placebo or a standard treatment in people with atopic eczema.
Search methods: We searched the following databases up to July 2015: the Cochrane Skin Group Specialised Register, CENTRAL in the Cochrane Library (Issue 7, 2015), MEDLINE (from 1946), EMBASE (from 1974), LILACS (from 1982), Web of Science™ (from 2005), the Global Resource of EczemA Trials (GREAT database), and five trials databases. We searched abstracts from recent European and North American allergy meetings and checked the references of included studies and review articles for further references to relevant trials.
Selection criteria: Randomised controlled trials (RCTs) of specific allergen immunotherapy that used standardised allergen extracts in people with AE.
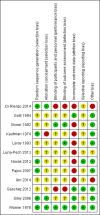
Data collection and analysis: Two authors independently undertook study selection, data extraction (including adverse effects), assessment of risk of bias, and analyses. We used standard methodological procedures expected by Cochrane.
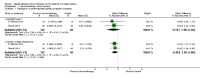
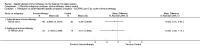
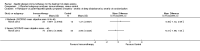
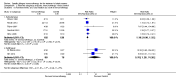
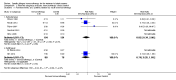
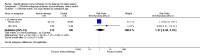
Main results: We identified 12 RCTs for inclusion in this review; the total number of participants was 733. The interventions included SIT in children and adults allergic to either house dust mite (10 trials), grass pollen, or other inhalant allergens (two trials). They were administered subcutaneously (six trials), sublingually (four trials), orally, or intradermally (two trials). Overall, the risk of bias was moderate, with high loss to follow up and lack of blinding as the main methodological concern.Our primary outcomes were 'Participant- or parent-reported global assessment of disease severity at the end of treatment'; 'Participant- or parent-reported specific symptoms of eczema, by subjective measures'; and 'Adverse events, such as acute episodes of asthma or anaphylaxis'. SCORing Atopic Dermatitis (SCORAD) is a means of measuring the effect of atopic dermatitis by area (A); intensity (B); and subjective measures (C), such as itch and sleeplessness, which we used.For 'Participant- or parent-reported global assessment of disease severity at the end of treatment', one trial (20 participants) found improvement in 7/9 participants (78%) treated with the SIT compared with 3/11 (27%) treated with the placebo (risk ratio (RR) 2.85, 95% confidence interval (CI) 1.02 to 7.96; P = 0.04). Another study (24 participants) found no difference: global disease severity improved in 8/13 participants (62%) treated with the SIT compared with 9/11 (81%) treated with the placebo (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38). We did not perform meta-analysis because of high heterogeneity between these two studies. The quality of the evidence was low.For 'Participant- or parent-reported specific symptoms of eczema, by subjective measures', two trials (184 participants) did not find that the SIT improved SCORAD part C (mean difference (MD) -0.74, 95% CI -1.98 to 0.50) or sleep disturbance (MD -0.49, 95% CI -1.03 to 0.06) more than placebo. For SCORAD part C itch severity, these two trials (184 participants) did not find that the SIT improved itch (MD -0.24, 95% CI -1.00 to 0.52). One other non-blinded study (60 participants) found that the SIT reduced itch compared with no treatment (MD -4.20, 95% CI -3.69 to -4.71) and reduced the participants' overall symptoms (P < 0.01), but we could not pool these three studies due to high heterogeneity. The quality of the evidence was very low.Seven trials reported systemic adverse reactions: 18/282 participants (6.4%) treated with the SIT had a systemic reaction compared with 15/210 (7.1%) with no treatment (RR 0.78, 95% CI 0.41 to 1.49; the quality of the evidence was moderate). The same seven trials reported local adverse reactions: 90/280 participants (32.1%) treated with the SIT had a local reaction compared with 44/204 (21.6%) in the no treatment group (RR 1.27, 95% CI 0.89 to 1.81). As these had the same study limitations, we deemed the quality of the evidence to also be moderate.Of our secondary outcomes, there was a significant improvement in 'Investigator- or physician-rated global assessment of disease severity at the end of treatment' (six trials, 262 participants; RR 1.48, 95% CI 1.16 to 1.88). None of the studies reported our secondary outcome 'Parent- or participant-rated eczema severity assessed using a published scale', but two studies (n = 184), which have been mentioned above, used SCORAD part C, which we included as our primary outcome 'Participant- or parent-reported specific symptoms of eczema, by subjective measures'.Our findings were generally inconclusive because of the small number of studies. We were unable to determine by subgroup analyses a particular type of allergen or a particular age or level of disease severity where allergen immunotherapy was more successful. We were also unable to determine whether sublingual immunotherapy was associated with more local adverse reactions compared with subcutaneous immunotherapy.
Authors' conclusions: Overall, the quality of the evidence was low. The low quality was mainly due to the differing results between studies, lack of blinding in some studies, and relatively few studies reporting participant-centred outcome measures. We found limited evidence that SIT may be an effective treatment for people with AE. The treatments used in these trials were not associated with an increased risk of local or systemic reactions. Future studies should use high quality allergen formulations with a proven track record in other allergic conditions and should include participant-reported outcome measures.
Conflict of interest statement
Herman Tam: nothing to declare. Moises A Calderon: nothing to declare. Logan Manikam: nothing to declare. Helen Nankervis: nothing to declare. Ignacio García Núñez: nothing to declare. Hywel C Williams: nothing to declare. Stephen Durham: "I have received research funding for immunotherapy trials in hay fever (but not eczema) via Imperial College from ALK‐Abelló, Denmark; Merck, USA; and BioTech Tools, Belgium; all are manufacturers of allergy vaccines (research in relation to vaccines for hay fever, not for eczema). I have acted as a paid advisor for Merck, USA, a manufacturer of allergy vaccines (in relation to allergy vaccines for hay fever, not for eczema). I have received consultancy fees via Imperial College from Circassia, UK; Stallergenes, France; and Biomay, Austria (in relation to vaccines for hay fever, not for eczema)." Robert J Boyle: nothing to declare.
Figures
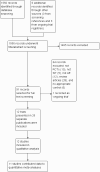











Update of
References
References to studies included in this review
Di Rienzo 2014 {published data only}
-
- Rienzo V, Cadario G, Grieco T, Galluccio AG, Caffarelli C, Liotta, et al. Sublingual immunotherapy in mite‐sensitized children with atopic dermatitis: A randomized, open, parallel‐group study. Annals of Allergy, Asthma & Immunology 2014;113(6):671‐3. [PUBMED: 25304342] - PubMed
-
- EudraCT 2008‐000196‐23. Efficacy of sublingual immunotherapy with HDM mix extract (Der p and Der f) (SLITone) in pediatric subjects with mild‐to‐moderate atopic eczema (AE) and sensitization to HDM (SPT positive) ( SLO‐AD‐1 Italy). www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐000196‐23/IT (accessed 1 December 2015).
Galli 1994 {published data only}
-
- Galli E, Chini L, Nardi S, Benincori N, Panei P, Fraioli G, et al. Use of a specific oral hyposensitization therapy to Dermatophagoides pteronyssinus in children with atopic dermatitis. Allergologia et immunopathologia 1994;22(1):18‐22. [MEDLINE: ] - PubMed
Glover 1992 {published data only}
-
- Glover MT, Atheron DJ. A double‐blind controlled trial of hyposensitization to the house dust mite in childhood atopic eczema. British Journal of Dermatology 1991;125(Suppl s38):87.
-
- Glover MT, Atherton DJ. A double‐blind controlled trial of hyposensitization to Dermatophagoides pteronyssinus in children with atopic eczema. Clinical & Experimental Allergy 1992;22(4):440‐6. [MEDLINE: ] - PubMed
Kaufman 1974 {published data only}
-
- Kaufman HS, Roth HL. Hyposensitization with alum precipitated extracts in atopic dermatitis: A placebo‐controlled study. Annals of Allergy 1974;32(6):321‐30. [MEDLINE: ] - PubMed
Leroy 1993 {published data only}
-
- Leroy B, Lachapelle JM, Jacquemin MG, Saint‐Remy JM. Immunotherapy of atopic dermatitis by injections of antigen‐antibody complexes. Dermatology 1993;186(4):276‐7. [MEDLINE: ] - PubMed
-
- Leroy BP, Boden G, Jacquemin MG, Lachapelle JM, Saint‐Remy JM. Allergen‐antibody complexes in the treatment of atopic dermatitis: preliminary results of a double‐blind placebo‐controlled study. Acta Dermato‐Venereologica. Supplementum 1992;176:129‐31. [MEDLINE: ] - PubMed
-
- Leroy BP, Boden G, Lachapelle JM, Jacquemin MG, Saint‐Remy JM. A novel therapy for atopic dermatitis with allergen‐antibody complexes: A double‐blind, placebo‐controlled study. Journal of the American Academy of Dermatology 1993;28(2 Pt 1):232‐9. [MEDLINE: ] - PubMed
Luna‐Pech 2013 {published data only}
-
- Luna‐Pech JA, Newton‐Sanchez OA, Torres‐Mendoza BM, Garcia‐Cobas CY. Efficacy of sublingual immunotherapy in the severity of atopic dermatitis in children with allergic sensitization to dermatophagoides pteronyssinus. 2013 Annual Meeting of the American College of Allergy, Asthma and Immunology Baltimore, MD United States. Annals of Allergy, Asthma & Immunology 2013;111(5 Suppl 1):A8. [EMBASE: 71306835]
Novak 2012 {published and unpublished data}
-
- Novak N, Bieber T, Hoffman M, Folster‐Holst M, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen‐specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. Journal of Allergy & Clinical Immunology 2012;130(4):925‐31. [MEDLINE: ] - PubMed
-
- Novak N, Zuberbier T, Sager A. Efficacy and safety of a depigmented polymerised mite extract in patients suffering from atopic eczema with clinical relevant IgE‐mediated sensitisation against house dust mites. 30th Congress of the European Academy of Allergy and Clinical Immunology Istanbul Turkey. Conference Start: 20110611 Conference End: 20110615. Allergy: European Journal of Allergy and Clinical Immunology 2011;66(Suppl s94):103. [EMBASE: 70640604]
Pajno 2007 {published data only}
-
- Pajno G, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite‐sensitized children with atopic dermatitis: A randomized, double‐blind, placebo‐controlled study. Journal of Allergy & Clinical Immunology 2007;120(1):164‐70. [MEDLINE: ] - PubMed
-
- Pajno G, Vita D, Caminiti L, Arrigo T, Lombardo F, Barberio G. House dust mite sublingual immunotherapy (SLIT) for atopic dermatitis (AD) a randomized controlled trial. Journal of Allergy & Clinical Immunology 2006;117(2 Suppl 1):S233.
-
- Pajno GB, Caminiti L, Vita D, Profazio C. Sublingual House Dust Mite (hdm) Immunotherapy, In Children With Extrinsic Allergic Form Of Atopic Dermatitis. A Randomized Controlled Trial On Prevention Of Appearance Of Asthma Or Rhinitis. 2010 American Academy of Allergy, Asthma and Immunology, AAAAI Annual Meeting New Orleans, LA United States. 26 February ‐ 2 March 2010. Journal of Allergy & Clinical Immunology 2010;125(2 Suppl 1):AB236. [EMBASE: 70156028]
-
- Passalacqua G, Pajno G. Long‐term prevention of asthma/rhinitis in children with atopic dermatitis 4 years after stopping sublingual immunotherapy. Allergy: European Journal of Allergy & Clinical Immunology 2012;67(Suppl 96):89. [EMBASE: 71109979]
Qin 2014 {published data only}
-
- NCT01471119. Sublingual Immunotherapy in Patients With Atopic Dermatitis. clinicaltrials.gov/ct2/show/NCT01471119 (accessed 1 December 2015).
-
- Qin YE, Mao JR, Sang YC, Li WX. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. International Journal of Dermatology 2014;53(5):650‐655. [MEDLINE: ] - PubMed
Sanchez 2012 {published and unpublished data}
Silny 2006 {published data only}
-
- Silny W, Czarnecka‐Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis ‐ Results of a double‐blind, placebo‐controlled study [Spezifische immuntherapie bei der behandlung von patienten mit atopischer dermatitis ‐ Ergebnisse einer plazebokontrollierten doppelblindstudie]. Allergologie 2006;29(5):171‐83. [EMBASE: 2006255579]
-
- Silny W, Czarnecka‐Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis ‐ results of double blind placebo controlled trial. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):155.
-
- Silny W, Czarnecka‐Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis‐‐results of double blind placebo controlled study. Polski Merkuriusz Lekarski 2006;21(126):558‐65. [MEDLINE: ] - PubMed
-
- Silny W, Czarnecka‐Operacz M, Silny P. Effectiveness of specific immunotherapy in the treatment of children and youngsters suffering from atopic dermatitis. Part III. Serum concentrations of selected immunologic parameters. Wiadomości Lekarskie 2005;58(5‐6):287‐94. [MEDLINE: ] - PubMed
-
- Silny W, Czarnecka‐Operacz M, Silny P. Efficacy of specific immunotherapy in the treatment of children and youngsters suffering from atopic dermatitis. Part I. Evaluation of clinical score. Wiadomości Lekarskie 2005;58(1‐2):47‐55. [MEDLINE: ] - PubMed
Warner 1978 {published and unpublished data}
-
- Warner JO, Price JF, Soothill JF, Hey EN. Controlled trial of hyposensitisation to dermatophagoides pteronyssinus in children with asthma. Lancet 1978;2(8096):912‐5. [MEDLINE: ] - PubMed
References to studies excluded from this review
Ariano 2009 {published data only}
-
- Ariano R, Incorvaia C, Grutta S, Marcucci F, Pajno G, Sensi L, et al. Safety of sublingual immunotherapy started during the pollen season. Current Medical Research & Opinion 2009;25(1):103‐7. [MEDLINE: ] - PubMed
Brunetti 2005 {published data only}
-
- Brunetti L, Francavilla R, Tesse R, Fiermonte P, Dambra P, Massagli M, et al. Effects of oral bacterial immunotherapy in children with atopic eczema/dermatitis syndrome: a pilot study. Biodrugs 2005;19(6):393‐9. [MEDLINE: ] - PubMed
Businco 1997 {published data only}
-
- Businco L. Early treatment of the atopic child: first results of the clinical trial. Pediatric Pulmonology ‐ Supplement 1997;16:73. [MEDLINE: ] - PubMed
Bussman 2007 {published data only}
-
- Bussmann C, Novak N. Allergen‐Specific Immunotherapy in Patients With Atopic Dermatitis?. Allergologie 2007;30(11):405‐10.
Cadario 2007 {published data only}
-
- Cadario G, Galluccio AG, Pezza M, Appino A, Milani M, Pecora S, et al. Sublingual immunotherapy efficacy in patients with atopic dermatitis and house dust mites sensitivity: A prospective pilot study. Current Medical Research & Opinion 2007;23(10):2503‐6. [MEDLINE: ] - PubMed
Canonica 2009 {published data only}
-
- Canonica GW, Bousquet J, Casale T, Lockey RF, Baena‐Cagnani CE, Pawankar R, et al. Sub‐lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy: European Journal of Allergy and Clinical Immunology 2009;64(Suppl 91):1‐59. [EMBASE: 2010086368] - PubMed
Compalati 2010 {published data only}
-
- Compalati E, Massimo L, Anthi R, Elisa V, Giovanni P, Giorgio WC. Abstracts of the XXIX EAACI Congress of the European Academy of Allergy and Clinical Immunology. London, United Kingdom. June 5‐9, 2010. Allergy 2010;65(Suppl 92):1‐756. [EMBASE: 20553234] - PubMed
D'Souza 1973 {published data only}
-
- D'Souza MF, Pepys J, Wells ID, Tai E, Palmer F, Overell BG, et al. Hyposensitization with Dermatophagoides pteronyssinus in house dust allergy: a controlled study of clinical and immunological effects. Clinical Allergy 1973;3(2):177‐93. [MEDLINE: ] - PubMed
Darsow 2005 {published data only}
-
- Darsow U, Forer I, Ring J. Specific Immunotherapy in Atopic Eczema [Spezifische hyposensibilisierung bei atopischem ekzem]. Allergologie 2005;28(2):53‐61. [EMBASE: 2005116033]
Derkach 2015 {published data only}
-
- Derkach V, Slavyanskaya T. Combination Immunotropic Therapy of Atopic Dermatitis in Children: Cost‐Benefit Analysis. 2015 American Academy of Allergy, Asthma and Immunology, AAAAI Annual Meeting Houston, TX United States. Journal of Allergy & Clinical Immunology 2015;135(2 Suppl):AB265.
Finegold 2009 {published data only}
-
- Finegold I. Immunotherapy at the ACAAI Annual Meeting. 6th International Congress on Autoimmunity, 9‐13 September 2008, Porto, Portugal. Immunotherapy 2009;1(2):177‐9. [MEDLINE: ] - PubMed
Gendelman 2011 {published data only}
-
- Gendelman S, Lang DM. Specific immunotherapy in treating atopic dermatitis: A systematic review using the GRADE system. 2011 American Academy of Allergy, Asthma and Immunology, AAAAI Annual Meeting San Francisco, CA United States. 18‐22 March 2011. Journal of Allergy & Clinical Immunology 2011;127(2 Suppl 1):AB49. [EMBASE: 70358915]
Gendelman 2013 {published data only}
-
- Gendelman SR, Lang DM. Specific immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Annals of Allergy, Asthma & Immunology 2013;111(6):555‐61. [MEDLINE: ] - PubMed
Gendelman 2014 {published data only}
-
- Gendelman SR, Lang DM. Sublingual Immunotherapy in the Treatment of Atopic Dermatitis: a Systematic Review Using the GRADE System. Current Allergy & Asthma Reports 2014;15(2):1‐8. - PubMed
Gendelman 2015 {published data only}
-
- Gendelman S, Lang DM. Sublingual Immunotherapy in the Treatment of AtopicDermatitis: a Systematic Review Using the GRADE System. Current Allergy and Asthma Reports 2015;15(2):498. - PubMed
Horak 2009 {published data only}
-
- Horak F. Sublingual immunotherapy: Noticeable improvement after a few weeks [SLIT ‐ Spurbare besserung bereits nach wenigen wochen]. Atemwegs‐ und Lungenkrankheiten 2009;35(7):325. [EMBASE: 2009425237]
Incorvaia 2009 {published data only}
-
- Incorvaia C, Mauro M, Cappelletti T, Pravettoni C, Leo G, Riario‐Sforza GG. New applications for sublingual immunotherapy in allergy. Recent Patents on Inflammation & Allergy Drug Discovery 2009;3(2):113‐7. [MEDLINE: ] - PubMed
Jacquemin 1995 {published data only}
-
- Jacquemin MG, Saint‐Remy JM. Specific down‐regulation of anti‐allergen IgE and IgG antibodies in humans associated with injections of allergen‐specific antibody complexes. Therapeutic Immunology 1995;2(1):41‐52. [MEDLINE: ] - PubMed
Juji 2003 {published data only}
-
- Juji F, Kobayashi S, Ito S, Sugawara N, Kano H, Yasueda H, et al. Immunotherapy by Japanese cedar pollen in atopic dermatitis. Arerugi 2003;52(11):1081‐8. - PubMed
Larenas‐Linnemann 2008 {published data only}
-
- Larenas‐Linnemann D. Briefings from ACAAI 2008 annual meeting. The Annual Scientific Meeting of the American College of Allergy, Asthma and Immunology, Seattle, WA, USA, 6‐11 November, 2008. Therapy 2009;6(2):279‐83. [EMBASE: 2009459563]
Larenas‐Linnemann 2009 {published data only}
-
- Larenas‐Linnemann D. Certainties and doubts about sublingual and oral immunotherapy in children. Current Opinion in Allergy & Clinical Immunology 2009;9(6):558‐67. [MEDLINE: ] - PubMed
Lee 2015 {published data only}
Leung 2015 {published data only}
-
- Leung TN, Hon KL. Eczema therapeutics in children: what do the clinical trials say?. Hong Kong Medical Journal 2015;21(3):251‐260. [PUBMED: 25904389] - PubMed
Margona 2015 {published data only}
-
- Marogna M, Braidi C, Colombo C, Colombo F, Palumbo L, Compalati E. Can sublingual allergen immunotherapy for house dust mites influence the longterm evolution of severe atopic dermatitis and the progression to respiratory allergy? Results of an observational comparison with pharmacotherapy. 34th Congress of the European Academy of Allergy and Clinical Immunology Barcelona Spain. Allergy 2015;70(Suppl):461. [EMBASE: 72029742]
Mastrandrea 2000 {published data only}
-
- Mastrandrea F, Serio G, Minelli M, Minardi A, Scarcia G, Coradduzza G, et al. Specific sublingual immunotherapy in atopic dermatitis. Results of a 6‐year follow‐up of 35 consecutive patients. Allergologia et Immunopathologia 2000;28(2):54‐62. [MEDLINE: ] - PubMed
Melamed 2010 {published data only}
-
- Melamed IR, Robinson L, Heffron M. The Benefit of Montelukast in Atopic Dermatitis Induced by Food Allergies. 2010 American Academy of Allergy, Asthma and Immunology, AAAAI Annual Meeting New Orleans, LA United States. 26 February to 2 March 2010. Journal of Allergy & Clinical Immunology 2010;125(2 Suppl 1):AB93. [EMBASE: 70155473]
Mihara 2008 {published data only}
-
- Mihara S, Hide M. Ebm and future direction of allergen‐specific immunotherapy for atopic dermatitis. Arerugi ‐ Japanese Journal of Allergology 2008;57(5):499‐506. [MEDLINE: ] - PubMed
Minelli 2010 {published data only}
-
- Minelli M, Schiavino D, Musca F, Bruno ME, Falagiani P, Mistrello G, et al. Oral hyposensitization to nickel induces clinical improvement and a decrease in TH1 and TH2 cytokines in patients with systemic nickel allergy syndrome. International Journal of Immunopathology & Pharmacology 2010;23(1):193‐201. [MEDLINE: ] - PubMed
Mohapatra 2010 {published data only}
Nahm 2008 {published data only}
-
- Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen‐specific immunotherapy and a histamine‐immunoglobulin complex. International Archives of Allergy & Immunology 2008;146(3):235‐40. [MEDLINE: ] - PubMed
Niebuhr 2007 {published data only}
-
- Niebuhr M, Kapp A, Werfel T. Specific immunotherapy in the treatment of atopic dermatitis [Spezifische Immuntherapie Bei Der Behandlung Der Atopischen Dermatitis: Hautarzt]. Hautarzt 2007;58(3):232‐6. [MEDLINE: ] - PubMed
Niebuhr 2008 {published data only}
-
- Niebuhr M, Kapp A, Werfel T. Specific immunotherapy (SIT) in atopic dermatitis and food allergy [Spezifische Immuntherapie (SIT) Bei Atopischer Dermatitis Und Nahrungsmittelallergie]. Hautarzt 2008;59(7):544‐50. [MEDLINE: ] - PubMed
Noh 2000 {published data only}
-
- Noh GW, Lee KY. Interferon‐gamma induced desensitization igid for house dust mites: modulation of immune status from th2 to th1 using interferon‐gamma as a new therapeutic concept for atopic dermatitis. Annals of Allergy, Asthma & Immunology 2000;84:155.
Novak 2007 {published data only}
-
- Novak N. Allergen specific immunotherapy for atopic dermatitis. Current Opinion in Allergy & Clinical Immunology 2007;7(6):542‐6. [MEDLINE: ] - PubMed
Ong 2010 {published data only}
-
- Ong PY, Boguniewicz M. Investigational and unproven therapies in atopic dermatitis. Immunology & Allergy Clinics of North America 2010;30(3):425‐39. [MEDLINE: ] - PubMed
Ozdemir 2009 {published data only}
-
- Ozdemir C. An immunological overview of allergen specific immunotherapy ‐ Subcutaneous and sublingual routes. Therapeutic Advances in Respiratory Disease 2009;3(5):253‐62. [MEDLINE: ] - PubMed
Panzani 1995 {published data only}
-
- Panzani RC, Schiavino D, Nucera E, Pellegrino S, Fais G, Schinco G, et al. Oral hyposensitization to nickel allergy: preliminary clinical results. International Archives of Allergy & Immunology 1995;107(1‐3):251‐4. [MEDLINE: ] - PubMed
Passalacqua 2012 {published data only}
-
- Passalacqua G, Garelli V, Sclifo F, Canonica GW, Pajno G. Immunotherapy – 2082. Long term prevention of asthma and rhinitis in children with atopic dermatitis four year after discontinuation of sublingual immunotherapy. 2nd WAO International Scientific Conference, WISC 2012 Hyderabad India. 6‐9 February 2012. World Allergy Organization Journal 2013;6(Suppl 1):P163. [EMBASE: 71252379]
Pereira 2013 {published data only}
-
- Pereira AM. Efficacy of allergen‐specific immunotherapy for atopic dermatitis: A systematic review and meta‐analysis of randomized controlled trials. Revista Portuguesa De Imunoalergologia 2013;21(3):215‐6. [EMBASE: 2014073384] - PubMed
Petrova 2001 {published data only}
-
- Petrova SI, Berzhets VM, Al'banova VI, Bystritskaia TF, Petrova NS. Immunotherapy in the complex treatment of patients with atopic dermatitis with sensitization to house dust mites [Immunoterapia v kompleksnom lechenii bol'nykh atopicheskim dermatitom s sensibilizatsiei k kleshcham domashnei pyli]. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2001;Jan‐Feb(1):33‐6. [EMBASE: 11236499] - PubMed
Pons‐Guiraud 1986 {published data only}
-
- Pons‐Guiraud A. Value of Allerglobulin in the treatment of atopic dermatitis in children and young adults. A double‐blind randomized study [Interet de l'allerglobuline dans le traitement de la dermatite atopique de l'enfant et de l'adulte jeune. Etude randomisee en double aveugle]. Revue De Medecine Interne 1986;7(5):537‐42. [MEDLINE: ] - PubMed
Ring 1982 {published data only}
-
- Ring J. Successful hyposensitization treatment in atopic eczema: results of a trial in monozygotic twins. British Journal of Dermatology 1982;107(5):597‐602. [MEDLINE: ] - PubMed
Roos 2004 {published data only}
-
- Roos TC, Geuer S, Roos S, Brost H. Recent Advances in Treatment Strategies for Atopic Dermatitis. Drugs 2004;64(23):2639‐66. [MEDLINE: ] - PubMed
Schiavino 2006 {published data only}
-
- Schiavino D, Nucera E, Alonzi C, Buonomo A, Pollastrini E, Roncallo C, et al. A clinical trial of oral hyposensitization in systemic allergy to nickel. International Journal of Immunopathology & Pharmacology 2006;19(3):593‐600. [MEDLINE: ] - PubMed
Senti 2009 {published data only}
-
- Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, et al. Use of A‐type CpG oligodeoxynucleotides as an adjuvant in allergen‐specific immunotherapy in humans: a phase I/IIa clinical trial. Clinical & Experimental Allergy 2009;39(4):562‐70. [MEDLINE: ] - PubMed
Shi 2010 {published data only}
-
- Shi H, Wang X, Ren H, Zhuang Y. Effect analysis of the dust mite allergen specific, immunotherapy of chronic eczema. Chinese Journal of Dermatovenereology 2010;24:424‐6.
Slavyanskaya 2014 {published data only}
-
- Slavyanskaya T, Derkach V. Rationale for the use of multiagent immunotherapy in children with atopic dermatitis. 33rd Congress of the European Academy of Allergy and Clinical Immunology Copenhagen Denmark. Allergy 2014;69(Suppl s99):190. [EMBASE: 71612794]
Slavyanskaya 2014b {published data only}
-
- Slavyanskaya T, Derkach V. Clinical substantiation of immunocorrective therapy in children with atopic dermatitis. 2013 WAO Symposium on Immunotherapy and Biologics. Chicago, IL, USA 13‐14 December 2013. World Allergy Organization Journal 2014;7(Suppl 1):P21. [EMBASE: 71440390]
Smolkin 2000 {published data only}
-
- Smolkin I, Pampura A, Ceburkin A, Morozova O. Influence of early specific immunotherapy by house dust mite allergens on development of asthma in children with atopic dermatitis. European Respiratory Journal 2000;16(Suppl 31):310s.
Stiller 1993 {published data only}
-
- Stiller MJ, Shupak JL, Soter NA. The synthetic immunoregulatory pentapeptide thymopentein: an adjunctive treatment in severe atopic dermatitis. Journal of Investigative Dermatology 1993;100(4):520.
Stiller 1994 {published data only}
-
- Stiller MJ, Shupack JL, Kenny C, Jondreau L, Cohen DE, Soter NA. A double‐blind, placebo‐controlled clinical trial to evaluate the safety and efficacy of thymopentin as an adjunctive treatment in atopic dermatitis. Journal of the American Academy of Dermatology 1994;30(4):597‐602. [MEDLINE: ] - PubMed
Strannegard 1982 {published data only}
-
- Strannegård O, Strannegård IL, Kang K, Cooper KD, Hanifin JM. FcIgG receptor‐bearing lymphocytes and monoclonal antibody‐defined T cell subsets in atopic dermatitis: effect of treatment with thymopoietin pentapeptide (TP‐5). International Archives of Allergy & Applied Immunology 1982;69(3):238‐44. [MEDLINE: ] - PubMed
Tammaro 2009 {published data only}
-
- Tammaro A, Marco G, Persechino S, Narcisi A, Camplone G. Allergy to nickel: first results on patients administered with an oral hyposensitization therapy. International Journal of Immunopathology & Pharmacology 2009;22(3):837‐40. [MEDLINE: ] - PubMed
Tonnel 2004 {published data only}
-
- Tonnel AB, Scherpereel A, Douay B, Mellin B, Leprince D, Goldstein N, et al. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy 2004;59(5):491‐7. [MEDLINE: ] - PubMed
Van Wijk 2008 {published data only}
-
- Rijk RG. When to initiate immunotherapy in children with allergic disease? Lessons from the paediatric studies.. Current Opinion in Allergy & Immunology 2008;8(6):565‐70. [MEDLINE: ] - PubMed
Wen 1992 {published data only}
-
- Wen T, Wang E, Shen S, Jiang C, Tian R, Kang K, et al. Allergenic potency of SMU‐Df extract in comparison with VUS‐Df extract; and diagnosis and immunotherapy for atopic dermatitis and rhinitis with SMU‐Df extract in China. Arbeiten aus dem Paul‐Ehrlich‐Institut (Bundesamt für Sera und Impfstoffe) zu Frankfurt Am. 1992;85:217‐27. [MEDLINE: ] - PubMed
Werfel 2006 {published data only}
-
- Werfel T, Breuer K, Rueff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi‐centre, randomized, dose‐response study. Allergy 2006;61(2):202‐5. [MEDLINE: ] - PubMed
Werfel 2007 {published data only}
-
- Werfel T, Kapp A. Specific immunotherapy in atopic eczema. Allergy & Clinical Immunology International 2007;19(3):100‐3. [EMBASE: 2007512301]
Werfel 2008 {published data only}
-
- Werfel T. The role of specific immunotherapy (SIT) in atopic dermatitis. Drugs of Today 2008;44(Suppl B):47‐9. [MEDLINE: ] - PubMed
Zachariae 1985 {published data only}
-
- Zachariae H, Cramers M, Herlin T, Jensen J, Kragballe K, Ternowitz T, et al. Non‐specific immunotherapy and specific hyposensitization in severe atopic dermatitis. Acta Dermato‐Venereologica. Supplementum 1985;65(114):48‐54. [MEDLINE: ] - PubMed
Zheng 2011 {published data only}
-
- Zheng J. Clinical Study on Sublingual Immunotherapy for Allergens of Children with Atopic Dermatitis. Herbal Medicine 2011;17:149‐50.
Zolkipli 2014 {published data only}
-
- Zolkipli Z, Roberts G, Cornelius V, Clayton CB, Pearson S, Michaelis LJ, et al. Randomised controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. 33rd Congress of the European Academy of Allergy and Clinical Immunology Copenhagen Denmark 7–11 June 2014. Allergy 2014;69(Suppl s99):105. [EMBASE: 71612566] - PubMed
Zolkipli 2014b {published data only}
-
- Zolkipli Z, Roberts G, Kurukulaaratchy R, Michaelis LJ, Matthews S, Clayton CB, et al. O24‐Mite allergy prevention study. Clinical & Translational Allergy. 3rd Pediatric Allergy and Asthma Meeting, PAAM 2013 Athens Greece. 17‐19 October 2013 2014;4(Suppl 1):O24. [EMBASE: 71812057] - PubMed
Zolkipli 2015 {published data only}
-
- Zolkipli Z, Roberts G, Cornelius V, Clayton B, Pearson S, Michaelis L, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. Journal of Allergy & Clinical Immunology 2015 Jun 12 [Epub ahead of print]. [DOI: 10.1016/j.jaci.2015.04.045; PUBMED: 26073754] - DOI - PubMed
References to ongoing studies
NCT00310492 {published data only}
-
- NCT00310492. Multicenter, randomized, double‐blind, placebo‐controlled parallel group study to demonstrate the efficacy of a 12‐month subcutaneous specific immunotherapy with ALK‐depot SQ milbenmischung in patients with atopic dermatitis and proven IgE‐mediated sensitization to house dust mites. clinicaltrials.gov/show/NCT00310492 (accessed 18 Nov 2014).
Additional references
Abramson 2003
Akdis 2006
-
- Akdis CA, Akdis M, Bieber T, Bindslev‐Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy 2006;61(8):969‐987. [MEDLINE: ] - PubMed
Allam 2006
-
- Allam JP, Novak N. The pathophysiology of atopic eczema. Clinical & Experimental Dermatology 2006;31(1):89‐93. [MEDLINE: ] - PubMed
Anagnostou 2014
Bae 2013
-
- Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen‐specific immunotherapy for atopic dermatitis: A systematic review and meta‐analysis of randomized controlled trials. Journal of Allergy & Clinical Immunology 2013;132(1):110‐7. [MEDLINE: ] - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [MEDLINE: ] - PubMed
Boyle 2012
Bussmann 2007
-
- Bussmann C, Maintz L, Hart J, Allam JP, Vrtala S, Chen KW, et al. Clinical improvement and immunological changes in atopic dermatitis patients undergoing subcutaneous immunotherapy with a house dust mite allergoid: A pilot study. Clinical & Experimental Allergy 2007;37(9):1277‐85. [MEDLINE: ] - PubMed
Bussmann 2009
-
- Bussmann C, Bieber T, Novak N. Systemic therapeutic options for severe atopic dermatitis. Journal der Deutschen Dermatologischen Gesellschaft 2009;7(3):205‐19. [MEDLINE: ] - PubMed
Calderon 2007
Calderon 2010
Calderon 2011
Capristo 2004
-
- Capristo C, Romei I, Boner AL. Environmental prevention in atopic eczema dermatitis syndrome (AEDS) and asthma: Avoidance of indoor allergens. Allergy 2004;59(Suppl 78):53‐60. [MEDLINE: ] - PubMed
CSM report 1986
Dahl 2006
-
- Dahl R, Kapp A, Colombo G, Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. Journal of Allergy & Clinical Immunology 2006;118(2):434‐40. [PUBMED: 16890769 ] - PubMed
Darsow 2012
-
- Darsow U. Allergen‐specific immunotherapy for atopic eczema: updated. Current Opinion in Allergy & Clinical Immunology 2012;12(6):665‐9. [PUBMED: 22918221] - PubMed
Didier 2007
-
- Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, et al. Optimal dose, efficacy and safety of once‐daily sublingual immunotherapy with a 5‐grass pollen tablet for seasonal allergic rhinitis. Journal of Allergy & Clinical Immunology 2007;120(6):1338‐45. [PUBMED: 17935764 ] - PubMed
Durham 1999
-
- Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. New England Journal of Medicine 1999;341(7):468‐75. [MEDLINE: ] - PubMed
Egger 1997
Gustafsson 2000
-
- Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis ‐ A prospective follow‐up to 7 years of age. Allergy 2000;55(3):240‐5. [MEDLINE: ] - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org., Available from www.cochrane‐handbook.org.
Illi 2004
-
- Illi S, Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy & Clinical Immunology 2004;113(5):925‐31. [MEDLINE: ] - PubMed
Johansson 2004
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy & Clinical Immunology 2004;113(5):832‐6. [MEDLINE: ] - PubMed
Lewis‐Jones 1995
-
- Lewis‐Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): Initial validation and practical use. British Journal of Dermatology 1995;132(6):942‐9. [MEDLINE: ] - PubMed
Maintz 2007
-
- Maintz L, Novak N. Getting more and more complex: The pathophysiology of atopic eczema. European Journal of Dermatology 2007;17(4):267‐83. [MEDLINE: ] - PubMed
Odhiambo 2009
-
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase Three Study Group. Global variation in prevalence of eczema symptoms in children from ISAAC phase three. Journal of Allergy & Clinical Immunology 2009;124(6):1251‐8. [MEDLINE: ] - PubMed
Penagos 2008
-
- Penagos M, Passalacqua G, Compalati E, Baena‐Cagnani CE, Orozco S, Pedroza A, et al. Meta‐analysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest 2008;133(3):599‐609. [PUBMED: 17951626 ] - PubMed
Purvis 2005
-
- Purvis DJ, Thompson JM, Clark PM, Robinson E, Black PN, Wild CJ, et al. Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. British Journal of Dermatology 2005;152(4):742‐9. [MEDLINE: ] - PubMed
Rotiroti 2012
-
- Rotiroti G, Shamji M, Durham SR, Till SJ. Repeated low‐dose intradermal allergen injection suppresses allergen‐induced cutaneous late responses. Journal of Allergy & Clinical Immunology 2012;130(4):918‐24. [PUBMED: 22971521] - PubMed
Schäfer 1999
-
- Schäfer T, Heinrich J, Wjst M, Adam H, Ring J, Wichmann HE. Association between severity of atopic eczema and degree of sensitization to aeroallergens in schoolchildren. Journal of Allergy & Clinical Immunology 1999;104(6):1280‐40. [MEDLINE: ] - PubMed
Tam 2013
-
- Tam H, Calderon MA, Boyle RJ. Efficacy of allergen‐specific immunotherapy for patients with atopic dermatitis. Journal of Allergy & Clinical Immunology 2013;132(4):1012‐3. [MEDLINE: ] - PubMed
Warner 2001
-
- Warner JO, ETAC Study Group, Early Treatment of the Atopic Child. A double‐blinded, randomized, placebo‐controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months' treatment and 18 months' post‐treatment follow‐up. Journal of Allergy & Clinical Immunology 2001;108(6):929‐37. [MEDLINE: ] - PubMed
Williams 2006
-
- Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. Journal of Allergy & Clinical Immunology 2006;118(1):209‐13. [PUBMED: 16815157] - PubMed
Wilson 2005
-
- Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: Systematic review and meta‐analysis. Allergy 2005;60(1):4‐12. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

