Integrated multi-omics analyses reveal the biochemical mechanisms and phylogenetic relevance of anaerobic androgen biodegradation in the environment
- PMID: 26872041
- PMCID: PMC5029156
- DOI: 10.1038/ismej.2015.255
Integrated multi-omics analyses reveal the biochemical mechanisms and phylogenetic relevance of anaerobic androgen biodegradation in the environment
Abstract
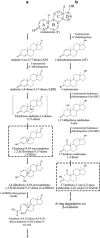
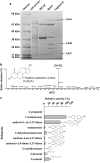
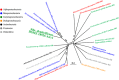
Steroid hormones, such as androgens, are common surface-water contaminants. However, literature on the ecophysiological relevance of steroid-degrading organisms in the environment, particularly in anoxic ecosystems, is extremely limited. We previously reported that Steroidobacter denitrificans anaerobically degrades androgens through the 2,3-seco pathway. In this study, the genome of Sdo. denitrificans was completely sequenced. Transcriptomic data revealed gene clusters that were distinctly expressed during anaerobic growth on testosterone. We isolated and characterized the bifunctional 1-testosterone hydratase/dehydrogenase, which is essential for anaerobic degradation of steroid A-ring. Because of apparent substrate preference of this molybdoenzyme, corresponding genes, along with the signature metabolites of the 2,3-seco pathway, were used as biomarkers to investigate androgen biodegradation in the largest sewage treatment plant in Taipei, Taiwan. Androgen metabolite analysis indicated that denitrifying bacteria in anoxic sewage use the 2,3-seco pathway to degrade androgens. Metagenomic analysis and PCR-based functional assays showed androgen degradation in anoxic sewage by Thauera spp. through the action of 1-testosterone hydratase/dehydrogenase. Our integrative 'omics' approach can be used for culture-independent investigations of the microbial degradation of structurally complex compounds where isotope-labeled substrates are not easily available.
Figures




References
-
- Adams MA, Teeter JH, Katz Y, Johnson PB. (1987). Sex-pheromones of the sea lamprey (Petromyzon marinus - steroid studies. J Chem Ecol 13: 387–395. - PubMed
-
- Andersen H, Siegrist H, Halling-Sorensen B, Ternes TA. (2003). Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37: 4021–4026. - PubMed
-
- Baronti C, Curini R, D'Ascenzo G, Di Corcia A, Gentili A, Samperi R. (2000). Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol 34: 5059–5066.
-
- Bhandare R, Calabro M, Coschigano PW. (2006). Site-directed mutagenesis of the Thauera aromatica strain T1 tutE tutFDGH gene cluster. Biochem Biophys Res Commun 346: 992–998. - PubMed
-
- Bortone SA, Davis WP, Bundrick CM. (1989). Morphological and behavioral characters in mosquitofish as potential bioindication of exposure to kraft mill effluent. Bull Environ Contam Toxicol 43: 370–377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

