Holistic Evaluation of Quality Consistency of Ixeris sonchifolia (Bunge) Hance Injectables by Quantitative Fingerprinting in Combination with Antioxidant Activity and Chemometric Methods
- PMID: 26872364
- PMCID: PMC4752467
- DOI: 10.1371/journal.pone.0148878
Holistic Evaluation of Quality Consistency of Ixeris sonchifolia (Bunge) Hance Injectables by Quantitative Fingerprinting in Combination with Antioxidant Activity and Chemometric Methods
Abstract
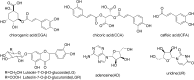
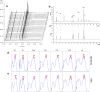
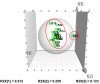
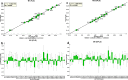
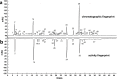
A widely used herbal medicine, Ixeris sonchifolia (Bge.) Hance Injectable (ISHI) was investigated for quality consistency. Characteristic fingerprints of 23 batches of the ISHI samples were generated at five wavelengths and evaluated by the systematic quantitative fingerprint method (SQFM) as well as simultaneous analysis of the content of seven marker compounds. Chemometric methods, i.e., support vector machine (SVM) and principal component analysis (PCA) were performed to assist in fingerprint evaluation of the ISHI samples. Qualitative classification of the ISHI samples by SVM was consistent with PCA, and in agreement with the quantitative evaluation by SQFM. In addition, the antioxidant activities of the ISHI samples were determined by both the off-line and on-line DPPH (2, 2-diphenyl-1-picryldrazyl) radical scavenging assays. A fingerprint-efficacy relationship linking the chemical components and in vitro antioxidant activity was established and validated using the partial least squares (PLS) and orthogonal projection to latent structures (OPLS) models; and the online DPPH assay further revealed those components that had position contribution to the total antioxidant activity. Therefore, the combined use of the chemometric methods, quantitative fingerprint evaluation by SQFM, and multiple marker compound analysis in conjunction with the assay of antioxidant activity provides a powerful and holistic approach to evaluate quality consistency of herbal medicines and their preparations.
Conflict of interest statement
Figures
References
-
- World Health Organization (1991) Guidelines for the Assessment of Herbal Medicines. Available: http://apps.who.int/iris/bitstream/10665/58865/1/WHO_TRM_91.4.pdf?ua=1. Accessed 7 March 2015.
-
- Food and Drug Administration (2004) Guidance for Industry Botanical Drug Product. Available:http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati.... Accessed 7 March 2015.
-
- European Medicines Agency (2010) Guideline on declaration of herbal substances and herbal preparations in herbal medicinal products /traditional herbal medicinal products. Available:http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 7 March 2015.
-
- Benzi G, Adriana C (1997) Herbal Medicines in European Regulation. Pharmacol Res 35: 355–362. - PubMed
-
- Information Office of the State Council of the People's Republic of China (2008) Status Quo of Drug Supervision in China. Available: http://eng.sfda.gov.cn/WS03/CL0757/62153.html. Accessed 7 March 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

