Copper and protons directly activate the zinc-activated channel
- PMID: 26872532
- PMCID: PMC5119521
- DOI: 10.1016/j.bcp.2016.02.004
Copper and protons directly activate the zinc-activated channel
Abstract
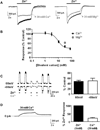
The zinc-activated channel (ZAC) is a cationic ion channel belonging to the superfamily of Cys-loop receptors, which consists of pentameric ligand-gated ion channels. ZAC is the least understood member of this family so in the present study we sought to characterize the properties of this channel further. We demonstrate that not only zinc (Zn(2+)) but also copper (Cu(2+)) and protons (H(+)) are agonists of ZAC, displaying potencies and efficacies in the rank orders of H(+)>Cu(2+)>Zn(2+) and H(+)>Zn(2+)>Cu(2+), respectively. The responses elicited by Zn(2+), Cu(2+) and H(+) through ZAC are all characterized by low degrees of desensitization. In contrast, currents evoked by high concentrations of the three agonists comprise distinctly different activation and decay components, with transitions to and from an open state being significantly faster for H(+) than for the two metal ions. The permeabilities of ZAC for Na(+) and K(+) relative to Cs(+) are indistinguishable, whereas replacing all of extracellular Na(+) and K(+) with the divalent cations Ca(2+) or Mg(2+) results in complete elimination of Zn(2+)-activated currents at both negative and positive holding potentials. This indicates that ZAC is non-selectively permeable to monovalent cations, whereas Ca(2+) and Mg(2+) inhibit the channel. In conclusion, this is the first report of a Cys-loop receptor being gated by Zn(2+), Cu(2+) and H(+). ZAC could be an important mediator of some of the wide range of physiological functions regulated by or involving Zn(2+), Cu(2+) and H(+).
Keywords: Calcium block; Copper; Cys-loop ligand-gated ion channels; Protons; Zinc.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures







References
-
- Davies PA, Wang W, Hales TG, Kirkness EF. A novel class of ligand-gated ion channel is activated by Zn2+ . J. Biol. Chem. 2003;278:712–717. - PubMed
-
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009;8:733–750. - PubMed
-
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS036296/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- R01 MH097446/MH/NIMH NIH HHS/United States
- R21 AA017938/AA/NIAAA NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- NS054900/NS/NINDS NIH HHS/United States
- P01 NS054900/NS/NINDS NIH HHS/United States
- R21 MH106954/MH/NIMH NIH HHS/United States
- MH097446/MH/NIMH NIH HHS/United States
- T32 NS061764/NS/NINDS NIH HHS/United States
- R01 NS036296/NS/NINDS NIH HHS/United States
- R01 NS047478/NS/NINDS NIH HHS/United States
- NS061764/NS/NINDS NIH HHS/United States
- R01 NS087662/NS/NINDS NIH HHS/United States
- R01 NS081986/NS/NINDS NIH HHS/United States
- NS047478/NS/NINDS NIH HHS/United States
- NS048045/NS/NINDS NIH HHS/United States
- AA017938/AA/NIAAA NIH HHS/United States
- R01 DA037170/DA/NIDA NIH HHS/United States
- R29 NS036296/NS/NINDS NIH HHS/United States
- R21 NS080064/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

