In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity
- PMID: 26872694
- PMCID: PMC4846978
- DOI: 10.1016/j.immuni.2016.01.010
In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity
Abstract
According to in vitro assays, T cells are thought to kill rapidly and efficiently, but the efficacy and dynamics of cytotoxic T lymphocyte (CTL)-mediated killing of virus-infected cells in vivo remains elusive. We used two-photon microscopy to quantify CTL-mediated killing in mice infected with herpesviruses or poxviruses. On average, one CTL killed 2-16 virus-infected cells per day as determined by real-time imaging and by mathematical modeling. In contrast, upon virus-induced MHC class I downmodulation, CTLs failed to destroy their targets. During killing, CTLs remained migratory and formed motile kinapses rather than static synapses with targets. Viruses encoding the calcium sensor GCaMP6s revealed strong heterogeneity in individual CTL functional capacity. Furthermore, the probability of death of infected cells increased for those contacted by more than two CTLs, indicative of CTL cooperation. Thus, direct visualization of CTLs during killing of virus-infected cells reveals crucial parameters of CD8(+) T cell immunity.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


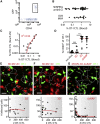


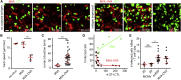


Comment in
-
Strength in Numbers: Visualizing CTL-Mediated Killing In Vivo.Immunity. 2016 Feb 16;44(2):207-8. doi: 10.1016/j.immuni.2016.01.026. Immunity. 2016. PMID: 26885849
References
-
- Altman J.D., Moss P.A., Goulder P.J., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Aramburu J., Garcia-Cózar F., Raghavan A., Okamura H., Rao A., Hogan P.G. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell. 1998;1:627–637. - PubMed
-
- Beltman J.B., Marée A.F.M., de Boer R.J. Analysing immune cell migration. Nat. Rev. Immunol. 2009;9:789–798. - PubMed
-
- Braun A., Worbs T., Moschovakis G.L., Halle S., Hoffmann K., Bölter J., Münk A., Förster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011;12:879–887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

