The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review
- PMID: 26873063
- PMCID: PMC4752749
- DOI: 10.1186/s12874-016-0111-9
The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review
Abstract
Background: Blinding is a pivotal method to avoid bias in randomised clinical trials. In blinded drug trials, experimental and control interventions are often designed to be matched, i.e. to appear indistinguishable. It is unknown how often matching procedures are inadequate, so we decided to systematically identify and analyse studies of matching quality in drug trials. Our primary objective was to assess the proportion of studies that concluded that the matching was inadequate; our secondary objective was to describe mechanisms for inadequate matching.
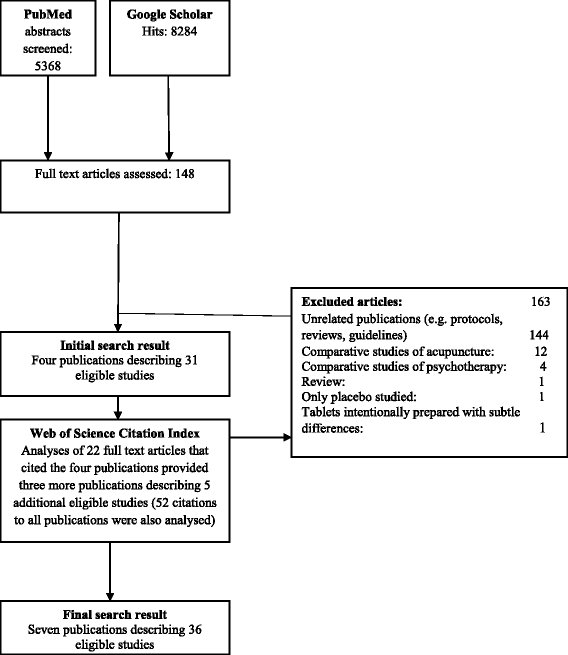
Methods: Systematic review. We searched PubMed, Google Scholar and Web of Science Citation Index for studies that assessed whether supposedly indistinguishable interventions (experimental and control) in randomized clinical drug trials could be distinguished based on physical properties (e.g. appearance or smell). Two persons decided on study eligibility and extracted data independently. Our primary analysis was based on the conclusions of each study. In supportive analyses, we defined a low and a high threshold for inadequate matching. We summarised results qualitatively.
Results: We included studies of 36 trials, of which 28 (78%) were published before 1977. The studies differed considerably with regard to design, methodology and analysis. Sixteen of the 36 studies (44%) concluded inadequate matching. When we adapted high or low thresholds for inadequate matching, the number of trials with inadequate matching was reduced to 12 (33%) or increased to 26 (72%). Inadequate matching was concluded in 7 of 22 trials (32%) based on a defined cohort of trials. Inadequate matching was concluded in 9 of 14 trials (64%) which were not based on a trial cohort, and therefore at a higher risk of publication bias. The proportion of inadequate matching did not seem to depend on publication year. Typical mechanisms of inadequate matching were differences in taste or colour.
Conclusion: We identified matching quality studies of 36 randomized clinical drug trials. Sixteen of the 36 studies (44%) concluded inadequate matching. Few studies of matching quality in contemporary trials have been published, but show similar results as found for older trials. Inadequate matching in drug trials may be more prevalent than commonly believed.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources