Germs within Worms: Localization of Neorickettsia sp. within Life Cycle Stages of the Digenean Plagiorchis elegans
- PMID: 26873314
- PMCID: PMC4959474
- DOI: 10.1128/AEM.04098-15
Germs within Worms: Localization of Neorickettsia sp. within Life Cycle Stages of the Digenean Plagiorchis elegans
Abstract
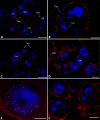
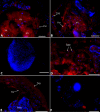
Neorickettsia spp. are bacterial endosymbionts of parasitic flukes (Digenea) that also have the potential to infect and cause disease (e.g., Sennetsu fever) in the vertebrate hosts of the fluke. One of the largest gaps in our knowledge of Neorickettsia biology is the very limited information available regarding the localization of the bacterial endosymbiont within its digenean host. In this study, we used indirect immunofluorescence microscopy to visualize Neorickettsia sp. within several life cycle stages of the digenean Plagiorchis elegans Individual sporocysts, cercariae, metacercariae, and adults of P. elegans naturally infected with Neorickettsia sp. were obtained from our laboratory-maintained life cycle, embedded, sectioned, and prepared for indirect immunofluorescence microscopy using anti-Neorickettsia risticiihorse serum as the primary antibody. Neorickettsiasp. was found within the tegument of sporocysts, throughout cercarial embryos (germ balls) and fully formed cercariae (within the sporocysts), throughout metacercariae, and within the tegument, parenchyma, vitellaria, uteri, testes, cirrus sacs, and eggs of adults. Interestingly, Neorickettsia sp. was not found within the ovarian tissue. This suggests that vertical transmission of Neorickettsia within adult digeneans occurs via the incorporation of infected vitelline cells into the egg rather than direct infection of the ooplasm of the oocyte, as has been described for other bacterial endosymbionts of invertebrates (e.g.,Rickettsia and Wolbachia).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Ultrastructure and localization of Neorickettsia in adult digenean trematodes provides novel insights into helminth-endobacteria interaction.Parasit Vectors. 2017 Apr 13;10(1):177. doi: 10.1186/s13071-017-2123-7. Parasit Vectors. 2017. PMID: 28407790 Free PMC article.
-
The numbers game: quantitative analysis of Neorickettsia sp. propagation through complex life cycle of its digenean host using real-time qPCR.Parasitol Res. 2016 Jul;115(7):2779-88. doi: 10.1007/s00436-016-5027-0. Epub 2016 Apr 4. Parasitol Res. 2016. PMID: 27041341
-
Laboratory maintenance of the bacterial endosymbiont, Neorickettsia sp., through the life cycle of a digenean, Plagiorchis elegans.Exp Parasitol. 2015 Oct;157:78-83. doi: 10.1016/j.exppara.2015.06.015. Epub 2015 Jul 6. Exp Parasitol. 2015. PMID: 26160679 Free PMC article.
-
Neorickettsial endosymbionts of the digenea: diversity, transmission and distribution.Adv Parasitol. 2012;79:253-97. doi: 10.1016/B978-0-12-398457-9.00003-2. Adv Parasitol. 2012. PMID: 22726644 Review.
-
Neorickettsia helminthoeca and salmon poisoning disease: a review.Vet J. 2011 Feb;187(2):165-73. doi: 10.1016/j.tvjl.2009.11.019. Epub 2009 Dec 30. Vet J. 2011. PMID: 20044285 Review.
Cited by
-
Ultrastructure and localization of Neorickettsia in adult digenean trematodes provides novel insights into helminth-endobacteria interaction.Parasit Vectors. 2017 Apr 13;10(1):177. doi: 10.1186/s13071-017-2123-7. Parasit Vectors. 2017. PMID: 28407790 Free PMC article.
-
Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal.Parasit Vectors. 2019 Oct 22;12(1):495. doi: 10.1186/s13071-019-3742-y. Parasit Vectors. 2019. PMID: 31640746 Free PMC article.
-
Real-Time PCR Differential Detection of Neorickettsia findlayensis and N. risticii in Cases of Potomac Horse Fever.J Clin Microbiol. 2022 Jul 20;60(7):e0025022. doi: 10.1128/jcm.00250-22. Epub 2022 Jun 13. J Clin Microbiol. 2022. PMID: 35695520 Free PMC article.
-
Isolation and Molecular Analysis of a Novel Neorickettsia Species That Causes Potomac Horse Fever.mBio. 2020 Feb 25;11(1):e03429-19. doi: 10.1128/mBio.03429-19. mBio. 2020. PMID: 32098825 Free PMC article.
-
Elevated prevalence of Helicobacter species and virulence factors in opisthorchiasis and associated hepatobiliary disease.Sci Rep. 2017 Feb 15;7:42744. doi: 10.1038/srep42744. Sci Rep. 2017. PMID: 28198451 Free PMC article.
References
-
- Cribb TH, Bray RA, Littlewood DTJ, Pichelin SP, Herniou EA. 2001. The digenea, p 168–185. In Littlewood DTJ, Brary RA (ed), Interrelationships of the platyhelminthes. Taylor and Francis, London, United Kingdom.
-
- Wen B, Rikihisa Y, Yamamoto S, Kawabata N, Fuerst PA. 1996. Characterization of the SF agent, and Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol 46:149–154. doi:10.1099/00207713-46-1-149. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

