Consensus Paper: Towards a Systems-Level View of Cerebellar Function: the Interplay Between Cerebellum, Basal Ganglia, and Cortex
- PMID: 26873754
- PMCID: PMC5243918
- DOI: 10.1007/s12311-016-0763-3
Consensus Paper: Towards a Systems-Level View of Cerebellar Function: the Interplay Between Cerebellum, Basal Ganglia, and Cortex
Abstract
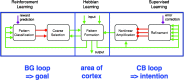
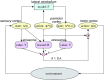
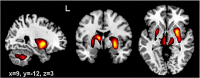
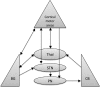
Despite increasing evidence suggesting the cerebellum works in concert with the cortex and basal ganglia, the nature of the reciprocal interactions between these three brain regions remains unclear. This consensus paper gathers diverse recent views on a variety of important roles played by the cerebellum within the cerebello-basal ganglia-thalamo-cortical system across a range of motor and cognitive functions. The paper includes theoretical and empirical contributions, which cover the following topics: recent evidence supporting the dynamical interplay between cerebellum, basal ganglia, and cortical areas in humans and other animals; theoretical neuroscience perspectives and empirical evidence on the reciprocal influences between cerebellum, basal ganglia, and cortex in learning and control processes; and data suggesting possible roles of the cerebellum in basal ganglia movement disorders. Although starting from different backgrounds and dealing with different topics, all the contributors agree that viewing the cerebellum, basal ganglia, and cortex as an integrated system enables us to understand the function of these areas in radically different ways. In addition, there is unanimous consensus between the authors that future experimental and computational work is needed to understand the function of cerebellar-basal ganglia circuitry in both motor and non-motor functions. The paper reports the most advanced perspectives on the role of the cerebellum within the cerebello-basal ganglia-thalamo-cortical system and illustrates other elements of consensus as well as disagreements and open questions in the field.
Keywords: Basal ganglia cerebellum anatomical link; Cerebellar motor and cognitive function; Movement disorders; Non-invasive brain stimulation; Nucleo-olivary inhibition; Parkinson’s disease tremor.
Figures
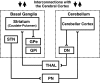
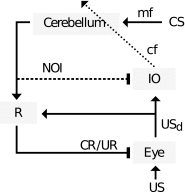
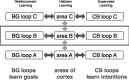
Comment in
-
The Known and Missing Links Between the Cerebellum, Basal Ganglia, and Cerebral Cortex.Cerebellum. 2017 Jun;16(3):753-755. doi: 10.1007/s12311-017-0850-0. Cerebellum. 2017. PMID: 28215041 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

