Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines
- PMID: 26873868
- PMCID: PMC4789831
- DOI: 10.1136/gutjnl-2015-311110
Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines
Abstract
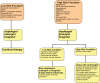
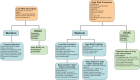
The risk of endoscopy in patients on antithrombotics depends on the risks of procedural haemorrhage versus thrombosis due to discontinuation of therapy. P2Y12 RECEPTOR ANTAGONISTS CLOPIDOGREL, PRASUGREL, TICAGRELOR: For low-risk endoscopic procedures we recommend continuing P2Y12 receptor antagonists as single or dual antiplatelet therapy (low quality evidence, strong recommendation); For high-risk endoscopic procedures in patients at low thrombotic risk, we recommend discontinuing P2Y12 receptor antagonists five days before the procedure (moderate quality evidence, strong recommendation). In patients on dual antiplatelet therapy, we suggest continuing aspirin (low quality evidence, weak recommendation). For high-risk endoscopic procedures in patients at high thrombotic risk, we recommend continuing aspirin and liaising with a cardiologist about the risk/benefit of discontinuation of P2Y12 receptor antagonists (high quality evidence, strong recommendation).
Warfarin: The advice for warfarin is fundamentally unchanged from British Society of Gastroenterology (BSG) 2008 guidance.
Direct oral anticoagulants doac: For low-risk endoscopic procedures we suggest omitting the morning dose of DOAC on the day of the procedure (very low quality evidence, weak recommendation); For high-risk endoscopic procedures, we recommend that the last dose of DOAC be taken ≥48 h before the procedure (very low quality evidence, strong recommendation). For patients on dabigatran with CrCl (or estimated glomerular filtration rate, eGFR) of 30-50 mL/min we recommend that the last dose of DOAC be taken 72 h before the procedure (very low quality evidence, strong recommendation). In any patient with rapidly deteriorating renal function a haematologist should be consulted (low quality evidence, strong recommendation).
Keywords: ENDOSCOPY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Comment in
-
Colonic stenting in patients on P2Y12 receptor antagonists and direct oral anticoagulants: are current BSG/ESGE guidelines practical?Gut. 2017 Feb;66(2):384. doi: 10.1136/gutjnl-2016-311781. Epub 2016 Mar 30. Gut. 2017. PMID: 27030508 No abstract available.
-
Author response to letter: colonic stenting in patients on P2Y12 receptor antagonists and direct oral anticoagulants-are current BSG/ESGE guidelines practical?Gut. 2017 Mar;66(3):560-561. doi: 10.1136/gutjnl-2016-312008. Epub 2016 Apr 28. Gut. 2017. PMID: 27196593 No abstract available.
References
-
- Agree Next Steps Consortium. The AGREE II Instrument (Electronic version), 2009. http://www.agreetrust.org
-
- Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous