Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension
- PMID: 26873938
- PMCID: PMC4836375
- DOI: 10.1096/fj.201500088R
Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension
Abstract
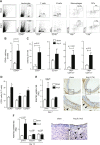
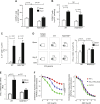
Recent studies have emphasized the role of perivascular inflammation in cardiovascular disease. We studied mechanisms of perivascular leukocyte infiltration in angiotensin II (Ang II)-induced hypertension and their links to vascular dysfunction. Chronic Ang II infusion in mice increased immune cell content of T cells (255 ± 130 to 1664 ± 349 cells/mg; P < 0.01), M1 and M2 macrophages, and dendritic cells in perivascular adipose tissue. In particular, the content of T lymphocytes bearing CC chemokine receptor (CCR) 1, CCR3, and CCR5 receptors for RANTES chemokine was increased by Ang II (CCR1, 15.6 ± 1.5% vs. 31 ± 5%; P < 0.01). Hypertension was associated with an increase in perivascular adipose tissue expression of the chemokine RANTES (relative quantification, 1.2 ± 0.2 vs. 3.5 ± 1.1; P < 0.05), which induced T-cell chemotaxis and vascular accumulation of T cells expressing the chemokine receptors CCR1, CCR3, and CCR5. Mechanistically, RANTES(-/-) knockout protected against vascular leukocyte, and in particular T lymphocyte infiltration (26 ± 5% in wild type Ang II vs. 15 ± 4% in RANTES(-/-)), which was associated with protection from endothelial dysfunction induced by Ang II. This effect was linked with diminished infiltration of IFN-γ-producing CD8(+) and double-negative CD3(+)CD4(-)CD8(-) T cells in perivascular space and reduced vascular oxidative stress while FoxP3(+) T-regulatory cells were unaltered. IFN-γ ex vivo caused significant endothelial dysfunction, which was reduced by superoxide anion scavenging. In a human cohort, a significant inverse correlation was observed between circulating RANTES levels as a biomarker and vascular function measured as flow-mediated dilatation (R = -0.3, P < 0.01) or endothelial injury marker von Willebrand factor (R = +0.3; P < 0.01). Thus, chemokine RANTES is important in the regulation of vascular dysfunction through modulation of perivascular inflammation.-Mikolajczyk, T. P., Nosalski, R., Szczepaniak, P., Budzyn, K., Osmenda, G., Skiba, D., Sagan, A., Wu, J., Vinh, A., Marvar, P. J., Guzik, B., Podolec, J., Drummond, G., Lob, H. E., Harrison, D. G., Guzik, T. J. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension.
Keywords: blood pressure; endothelial function; immune activation; superoxide; vascular inflammation.
© The Author(s).
Figures





References
-
- Margaritis M., Antonopoulos A. S., Digby J., Lee R., Reilly S., Coutinho P., Shirodaria C., Sayeed R., Petrou M., De Silva R., Jalilzadeh S., Demosthenous M., Bakogiannis C., Tousoulis D., Stefanadis C., Choudhury R. P., Casadei B., Channon K. M., Antoniades C. (2013) Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127, 2209–2221 - PubMed
-
- Gao Y. J. (2007) Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr. Pharm. Des. 13, 2185–2192 - PubMed
-
- Wu H., Ghosh S., Perrard X. D., Feng L., Garcia G. E., Perrard J. L., Sweeney J. F., Peterson L. E., Chan L., Smith C. W., Ballantyne C. M. (2007) T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038 - PubMed
-
- Takemori K., Gao Y. J., Ding L., Lu C., Su L. Y., An W. S., Vinson C., Lee R. M. (2007) Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension 49, 365–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

