The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection
- PMID: 26873979
- PMCID: PMC4783372
- DOI: 10.1093/jxb/erw016
The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection
Abstract
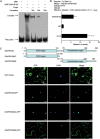
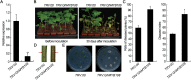
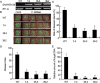
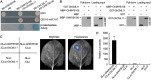
Accumulating evidence indicates that plant MYB transcription factors participate in defense against pathogen attack, but their regulatory targets and related signaling processes remain largely unknown. Here, we identified a defense-related MYB gene (GhMYB108) from upland cotton (Gossypium hirsutum) and characterized its functional mechanism. Expression of GhMYB108 in cotton plants was induced by Verticillium dahliae infection and responded to the application of defense signaling molecules, including salicylic acid, jasmonic acid, and ethylene. Knockdown of GhMYB108 expression led to increased susceptibility of cotton plants to V. dahliae, while ecotopic overexpression of GhMYB108 in Arabidopsis thaliana conferred enhanced tolerance to the pathogen. Further analysis demonstrated that GhMYB108 interacted with the calmodulin-like protein GhCML11, and the two proteins form a positive feedback loop to enhance the transcription of GhCML11 in a calcium-dependent manner. Verticillium dahliae infection stimulated Ca(2+) influx into the cytosol in cotton root cells, but this response was disrupted in both GhCML11-silenced plants and GhMYB108-silenced plants in which expression of several calcium signaling-related genes was down-regulated. Taken together, these results indicate that GhMYB108 acts as a positive regulator in defense against V. dahliae infection by interacting with GhCML11. Furthermore, the data also revealed the important roles and synergetic regulation of MYB transcription factor, Ca(2+), and calmodulin in plant immune responses.
Keywords: Calcium; MYB; Verticillium dahliae.; calmodulin; cotton; plant defense.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
-
- Bolek Y, El-Zik KM, Pepper AE, Bell AA, Magill CW, Thaxton PM, Reddy OUK. 2005. Mapping of verticillium wilt resistance genes in cotton. Plant Science 168, 1581–1590.
-
- Bouché N, Yellin A, Snedden WA, Fromm H. 2005. Plant-specific calmodulin-binding proteins. Annual Review of Plant Biology 56, 435–466. - PubMed
-
- Buscaill P, Rivas S. 2014. Transcriptional control of plant defence responses. Current Opinion in Plant Biology 20, 35–46. - PubMed
-
- Cai Y, He X, Mo J, Sun Q, Yang J, Liu J. 2009. Molecular research and genetic engineering of resistance to Verticillium wilt in cotton. African Journal of Biotechnology 8, 7363–7372.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

