Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans
- PMID: 26873992
- PMCID: PMC4777919
- DOI: 10.4049/jimmunol.1502478
Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans
Abstract
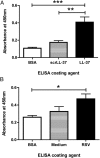
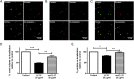
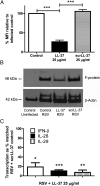
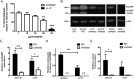
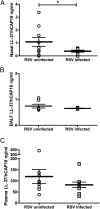
Respiratory syncytial virus (RSV) is a leading cause of respiratory tract infection in infants, causing significant morbidity and mortality. No vaccine or specific, effective treatment is currently available. A more complete understanding of the key components of effective host response to RSV and novel preventative and therapeutic interventions are urgently required. Cathelicidins are host defense peptides, expressed in the inflamed lung, with key microbicidal and modulatory roles in innate host defense against infection. In this article, we demonstrate that the human cathelicidin LL-37 mediates an antiviral effect on RSV by inducing direct damage to the viral envelope, disrupting viral particles and decreasing virus binding to, and infection of, human epithelial cells in vitro. In addition, exogenously applied LL-37 is protective against RSV-mediated disease in vivo, in a murine model of pulmonary RSV infection, demonstrating maximal efficacy when applied concomitantly with virus. Furthermore, endogenous murine cathelicidin, induced by infection, has a fundamental role in protection against disease in vivo postinfection with RSV. Finally, higher nasal levels of LL-37 are associated with protection in a healthy human adult RSV infection model. These data lead us to propose that cathelicidins are a key, nonredundant component of host defense against pulmonary infection with RSV, functioning as a first point of contact antiviral shield and having additional later-phase roles in minimizing the severity of disease outcome. Consequently, cathelicidins represent an inducible target for preventative strategies against RSV infection and may inform the design of novel therapeutic analogs for use in established infection.
Copyright © 2016 The Authors.
Figures




References
-
- Smyth R. L., Openshaw P. J. 2006. Bronchiolitis. Lancet 368: 312–322. - PubMed
-
- Nair H., Nokes D. J., Gessner B. D., Dherani M., Madhi S. A., Singleton R. J., O’Brien K. L., Roca A., Wright P. F., Bruce N., et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375: 1545–1555. - PMC - PubMed
-
- Krishnamoorthy N., Khare A., Oriss T. B., Raundhal M., Morse C., Yarlagadda M., Wenzel S. E., Moore M. L., Peebles R. S., Jr., Ray A., Ray P. 2012. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 18: 1525–1530. - PMC - PubMed
-
- Sigurs N., Aljassim F., Kjellman B., Robinson P. D., Sigurbergsson F., Bjarnason R., Gustafsson P. M. 2010. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 65: 1045–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

