Transdifferentiation of human endothelial progenitors into smooth muscle cells
- PMID: 26874281
- PMCID: PMC4763719
- DOI: 10.1016/j.biomaterials.2016.01.066
Transdifferentiation of human endothelial progenitors into smooth muscle cells
Abstract
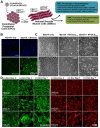
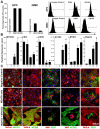
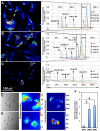
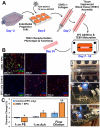
Access to smooth muscle cells (SMC) would create opportunities for tissue engineering, drug testing, and disease modeling. Herein we report the direct conversion of human endothelial progenitor cells (EPC) to induced smooth muscle cells (iSMC) by induced expression of MYOCD. The EPC undergo a cytoskeletal rearrangement resembling that of mesenchymal cells within 3 days post initiation of MYOCD expression. By day 7, the reprogrammed cells show upregulation of smooth muscle markers ACTA2, MYH11, and TAGLN by qRT-PCR and ACTA2 and MYH11 expression by immunofluorescence. By two weeks, they resemble umbilical artery SMC in microarray gene expression analysis. The iSMC, in contrast to EPC control, show calcium transients in response to phenylephrine stimulation and a contractility an order of magnitude higher than that of EPC as determined by traction force microscopy. Tissue-engineered blood vessels constructed using iSMC show functionality with respect to flow- and drug-mediated vasodilation and vasoconstriction.
Keywords: Direct reprogramming; Direct transdifferentiation; Myocardin; Smooth muscle cell differentiation; Tissue-engineered blood vessel.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- de Mel A, et al. A potential platform for developing 3D tubular scaffolds for paediatric organ development. J Mater Sci Mater Med. 2015;26:141. doi:10.1007/s10856-015-5477-4. - PubMed
-
- Ha JM, et al. Platelet-derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1. Biochem Biophys Res Commun. 2015 doi:10.1016/j.bbrc.2015.05.097. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi:10.1152/physrev.00041.2003. - PubMed
-
- Li S, Sims S, Jiao Y, Chow LH, Pickering JG. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338–348. - PubMed
-
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi:10.1152/physrev.00023.2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

