Human cartilage endplate permeability varies with degeneration and intervertebral disc site
- PMID: 26874969
- PMCID: PMC4779374
- DOI: 10.1016/j.jbiomech.2016.01.007
Human cartilage endplate permeability varies with degeneration and intervertebral disc site
Abstract
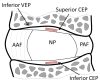
Despite the critical functions the human cartilage endplate (CEP) plays in the intervertebral disc, little is known about its structural and mechanical properties and their changes with degeneration. Quantifying these changes with degeneration is important for understanding how the CEP contributes to the function and pathology of the disc. Therefore the objectives of this study were to quantify the effect of disc degeneration on human CEP mechanical properties, determine the influence of superior and inferior disc site on mechanics and composition, and simulate the role of collagen fibers in CEP and disc mechanics using a validated finite element model. Confined compression data and biochemical composition data were used in a biphasic-swelling model to calculate compressive extrafibrillar elastic and permeability properties. Tensile properties were obtained by applying published tensile test data to an ellipsoidal fiber distribution. Results showed that with degeneration CEP permeability decreased 50-60% suggesting that transport is inhibited in the degenerate disc. CEP fibers are organized parallel to the vertebrae and nucleus pulposus and may contribute to large shear strains (0.1-0.2) and delamination failure of the CEP commonly seen in herniated disc tissue. Fiber-reinforcement also reduces CEP axial strains thereby enhancing fluid flux by a factor of 1.8. Collectively, these results suggest that the structure and mechanics of the CEP may play critical roles in the solute transport and disc mechanics.
Keywords: Biphasic; Cartilage endplate; Intervertebral disc; Permeability; Spine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures




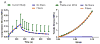


References
-
- Antoniou J, Goudsouzian N, Heathfield T, Winterbottom N, Steffen T, Poole A, Aebi M, Alini M. The Human Lumbar Endplate. Spine (Phila Pa 1976) 1996;21:1153–1161. - PubMed
-
- Armstrong CG, V, Mow C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. Journal of Bone and Joint Surgery. 1982;64:88–94. - PubMed
-
- Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transport in Porous Media. 2003;50:5–33.
-
- Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. Journal of Orthopedic Research. 1991;9:330–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

