A Crossover Design for Comparative Efficacy: A 36-Week Randomized Trial of Bevacizumab and Ranibizumab for Diabetic Macular Edema
- PMID: 26875003
- PMCID: PMC4988394
- DOI: 10.1016/j.ophtha.2015.11.021
A Crossover Design for Comparative Efficacy: A 36-Week Randomized Trial of Bevacizumab and Ranibizumab for Diabetic Macular Edema
Abstract
Purpose: To investigate the comparative efficacy of bevacizumab (Avastin) and ranibizumab (Lucentis; both Genentech, Inc, South San Francisco, CA) for diabetic macular edema (DME) using a crossover study design.
Design: Randomized, double-masked, 36-week, 3-period crossover clinical trial.
Participants: Fifty-six subjects with DME involving the center of the macula in one or both eyes.
Methods: Monthly intravitreous injections of bevacizumab (1.25 mg) or ranibizumab (0.3 mg).
Main outcome measures: Comparison of mean changes in visual acuity and central retinal thickness, tested using a linear mixed-effects model.
Results: Based on the linear mixed-effects model, the 3-month estimated mean improvement in visual acuity was 5.3 letters for bevacizumab and 6.6 letters for ranibizumab (difference, 1.3 letters; P = 0.039). Estimated change in optical coherence tomography (OCT) central subfield mean thickness (CSMT) was -89 μm for bevacizumab and -137 μm for ranibizumab (difference, 48 μm; P < 0.001). Incorporating cumulative treatment benefit, the model yielded a predicted 36-week (9-month) average improvement in visual acuity of 7.1 letters (95% confidence interval [CI], 5.0-9.2) for bevacizumab and 8.4 letters (95% CI, 6.3-10.5) for ranibizumab, and a change in OCT CSMT of -128 μm (95% CI, -155 to -100) for bevacizumab and -176 μm (95% CI, -202 to -149) for ranibizumab. There was no significant treatment-by-period interaction (i.e., treatment difference was constant in all 3 periods), nor was there a significant differential carryover effect from one period to the next.
Conclusions: This trial demonstrated a statistically significant but small relative clinical benefit of ranibizumab compared with bevacizumab for treatment of DME, using a markedly reduced sample size relative to a full comparative efficacy study. The effects on visual acuity and central retinal thickness for the 2 drugs are consistent with those reported at 1 year for the concurrent parallel-group trial by the Diabetic Retinopathy Clinical Research Network testing bevacizumab, ranibizumab, and aflibercept for DME. The 3-period crossover design allowed for meaningful and efficient comparison, suggesting that this approach may be useful for future comparative efficacy studies of anti-vascular endothelial growth factor drugs for DME.
Published by Elsevier Inc.
Conflict of interest statement
Figures
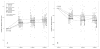

References
-
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. - PubMed
-
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. - PubMed
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

