Adaptive dosing of anticancer drugs in neonates: facilitating evidence-based dosing regimens
- PMID: 26875154
- PMCID: PMC4819938
- DOI: 10.1007/s00280-016-2975-0
Adaptive dosing of anticancer drugs in neonates: facilitating evidence-based dosing regimens
Abstract
Purpose: Selection of the most appropriate chemotherapy dosing regimens for neonates treated within the first weeks of life represents a significant clinical dilemma. Due to a lack of information relating to the clinical pharmacology of anticancer drugs in these challenging patients, current dosing guidelines are based on limited scientific rationale. In the current study, we investigate the utilisation of therapeutic drug monitoring approaches in neonates with localised hepatoblastoma, Wilms' tumour and stage 4S neuroblastoma, being treated with widely used anticancer drugs.
Methods: Plasma concentrations of cisplatin, vincristine, etoposide and carboplatin were quantified in two neonates being treated within the first 3 weeks of life and in a 32-week preterm infant treated at a gestational age of 40 weeks. Therapeutic drug monitoring was carried out where appropriate, based on the pharmacokinetic data obtained in conjunction with clinical response and toxicity.
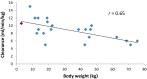
Results: Treatment of a child aged 2 weeks with a recommended cisplatin dose reduction for weight to 1.8 mg/kg resulted in achievement of unbound cisplatin plasma concentrations of 0.01-0.08 µg/mL, markedly lower than exposures previously reported in infants and older children. A dose increase to 2.7 mg/kg was implemented, leading to the achievement of levels more in-line with those previously reported. This increased dose level was well tolerated over six courses of treatment, resulting in a good response to cisplatin monotherapy and the patient remains in remission at 3.5 years. In contrast, a 50 % vincristine dose reduction for weight in a 3-week-old neonate resulted in plasma concentrations comparable to levels observed in older children, leading to successful treatment and continued remission at 2 years. In a third patient, etoposide and carboplatin clearance values normalised to body weight were comparable to those reported in older children, resulting in comparatively lower exposures following reduced dosing.
Conclusions: The current report provides unique data on the pharmacokinetics of several widely used anticancer drugs in neonates treated within the first few weeks of life. The provision of these data acts as a useful reference point to support future dosing decisions to be made by clinicians in the treatment of these challenging patients.
Keywords: Carboplatin; Chemotherapy; Cisplatin; Etoposide; Hepatoblastoma; Neonates; Neuroblastoma; Therapeutic drug monitoring; Vincristine; Wilms’ tumour.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

