Constructing modular and universal single molecule tension sensor using protein G to study mechano-sensitive receptors
- PMID: 26875524
- PMCID: PMC4753514
- DOI: 10.1038/srep21584
Constructing modular and universal single molecule tension sensor using protein G to study mechano-sensitive receptors
Abstract
Recently a variety of molecular force sensors have been developed to study cellular forces acting through single mechano-sensitive receptors. A common strategy adopted is to attach ligand molecules on a surface through engineered molecular tethers which report cell-exerted tension on receptor-ligand bonds. This approach generally requires chemical conjugation of the ligand to the force reporting tether which can be time-consuming and labor-intensive. Moreover, ligand-tether conjugation can severely reduce the activity of protein ligands. To address this problem, we developed a Protein G (ProG)-based force sensor in which force-reporting tethers are conjugated to ProG instead of ligands. A recombinant ligand fused with IgG-Fc is conveniently assembled with the force sensor through ProG:Fc binding, therefore avoiding ligand conjugation and purification processes. Using this approach, we determined that molecular tension on E-cadherin is lower than dsDNA unzipping force (nominal value: 12 pN) during initial cadherin-mediated cell adhesion, followed by an escalation to forces higher than 43 pN (nominal value). This approach is highly modular and potentially universal as we demonstrate using two additional receptor-ligand interactions, P-selectin &PSGL-1 and Notch &DLL1.
Figures
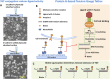
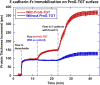

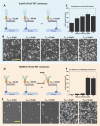
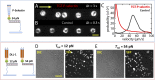
References
-
- Giancotti F. G. & Ruoslahti E. Transduction - Integrin signaling. Science 285, 1028–1032 (1999). - PubMed
-
- Takeichi M. Cadherin Cell-Adhesion Receptors as a Morphogenetic Regulator. Science 251, 1451–1455 (1991). - PubMed
-
- Artavanis-Tsakonas S., Rand M. D. & Lake R. J. Notch signaling: Cell fate control and signal integration in development. Science 284, 770–776 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

