Isatis indigotica root polysaccharides as adjuvants for an inactivated rabies virus vaccine
- PMID: 26875535
- PMCID: PMC7112441
- DOI: 10.1016/j.ijbiomac.2016.02.023
Isatis indigotica root polysaccharides as adjuvants for an inactivated rabies virus vaccine
Abstract
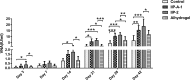
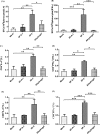
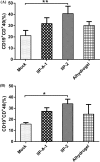
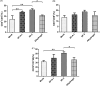
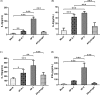
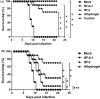
Adjuvants can enhance vaccine immunogenicity and induce long-term enhancement of immune responses. Thus, adjuvants are important for vaccine research. Polysaccharides isolated from select Chinese herbs have been demonstrated to possess various beneficial functions and excellent adjuvant abilities. In the present study, the polysaccharides IIP-A-1 and IIP-2 were isolated from Isatis indigotica root and compared with the common vaccine adjuvant aluminum hydroxide via intramuscular co-administration of inactivated rabies virus rCVS-11-G into mice. Blood was collected to determine virus neutralizing antibody (VNA) titers and B and T lymphocyte activation status. Inguinal lymph node samples were collected and used to measure B lymphocyte proliferation. Splenocytes were isolated, from which antigen-specific cellular immune responses were detected via ELISpot, ELISA and intracellular cytokine staining. The results revealed that both types of polysaccharides induce more rapid changes and higher VNA titers than aluminum hydroxide. Flow cytometry assays revealed that the polysaccharides activated more B lymphocytes in the lymph nodes and more B and T lymphocytes in the blood than aluminum hydroxide. Antigen-specific cellular immune responses showed that IIP-2 strongly induced T lymphocyte proliferation in the spleen and high levels of cytokine secretion from splenocytes, whereas aluminum hydroxide induced proliferation in only a small number of lymphocytes and the secretion of only small quantities of cytokines. Collectively, these data suggest that the polysaccharide IIP-2 exhibits excellent adjuvant activity and can enhance both cellular and humoral immunity.
Keywords: Adjuvant; Antibody titer; Cellular immunity; Polysaccharide; Rabies vaccine.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Adjuvant activity of PCP-II, a polysaccharide from Poria cocos, on a whole killed rabies vaccine.Virus Res. 2019 Sep;270:197638. doi: 10.1016/j.virusres.2019.06.001. Epub 2019 Jun 4. Virus Res. 2019. PMID: 31173772
-
Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines.Int J Biol Macromol. 2012 Apr 1;50(3):598-602. doi: 10.1016/j.ijbiomac.2012.01.035. Epub 2012 Feb 3. Int J Biol Macromol. 2012. PMID: 22326819
-
Incorporating B cell activating factor (BAFF) into the membrane of rabies virus (RABV) particles improves the speed and magnitude of vaccine-induced antibody responses.PLoS Negl Trop Dis. 2019 Nov 14;13(11):e0007800. doi: 10.1371/journal.pntd.0007800. eCollection 2019 Nov. PLoS Negl Trop Dis. 2019. PMID: 31725816 Free PMC article.
-
Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants.Carbohydr Polym. 2022 Jan 15;276:118739. doi: 10.1016/j.carbpol.2021.118739. Epub 2021 Oct 25. Carbohydr Polym. 2022. PMID: 34823775 Review.
-
Application prospect of polysaccharide in the development of vaccine adjuvants.Int J Biol Macromol. 2025 Mar;297:139845. doi: 10.1016/j.ijbiomac.2025.139845. Epub 2025 Jan 15. Int J Biol Macromol. 2025. PMID: 39824409 Review.
Cited by
-
Marburg virus-like particles by co-expression of glycoprotein and matrix protein in insect cells induces immune responses in mice.Virol J. 2017 Oct 25;14(1):204. doi: 10.1186/s12985-017-0869-3. Virol J. 2017. PMID: 29070075 Free PMC article.
-
Regulation of innate and adaptive immunity using herbal medicine: benefits for the COVID-19 vaccination.Acupunct Herb Med. 2022 Sep;2(3):196-206. doi: 10.1097/HM9.0000000000000046. Epub 2022 Dec 8. Acupunct Herb Med. 2022. PMID: 37808346 Free PMC article. Review.
-
Polysaccharide PCP-I isolated from Poria cocos enhances the immunogenicity and protection of an anthrax protective antigen-based vaccine.Hum Vaccin Immunother. 2020 Jul 2;16(7):1699-1707. doi: 10.1080/21645515.2019.1675457. Epub 2019 Dec 6. Hum Vaccin Immunother. 2020. PMID: 31809637 Free PMC article.
-
Immunomodulatory effects of Radix isatidis polysaccharides in vitro and in vivo.Exp Ther Med. 2021 Dec;22(6):1405. doi: 10.3892/etm.2021.10841. Epub 2021 Oct 4. Exp Ther Med. 2021. PMID: 34675998 Free PMC article.
-
Developments in Rabies Vaccines: The Path Traversed from Pasteur to the Modern Era of Immunization.Vaccines (Basel). 2023 Mar 29;11(4):756. doi: 10.3390/vaccines11040756. Vaccines (Basel). 2023. PMID: 37112668 Free PMC article. Review.
References
-
- Dietzschold B., Schnell M., Koprowski H. Pathogenesis of rabies. Curr. Top. Microbiol. Immunol. 2005;292:45–56. - PubMed
-
- Fu Z.F. Rabies and rabies research: past, present and future. Vaccine. 1997;15(Suppl):S20–S24. - PubMed
-
- World Health Organization, WHO Expert Consultation on Rabies, Second Report, World Health Organ. Tech. Rep. Ser., 2013, pp. 1–139 (back cover). - PubMed
-
- Rupprecht C.E. A tale of two worlds: public health management decisions in human rabies prevention. Clin. Infect. Dis. 2004;39:281–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

