Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: A randomized, double-blind, phase 3 study
- PMID: 26875540
- PMCID: PMC5067558
- DOI: 10.1111/1346-8138.13258
Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: A randomized, double-blind, phase 3 study
Abstract
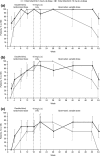
Tofacitinib is an oral Janus kinase inhibitor that is being investigated for psoriasis and psoriatic arthritis. Japanese patients aged 20 years or more with moderate to severe plaque psoriasis and/or psoriatic arthritis were double-blindly randomized 1:1 to tofacitinib 5 or 10 mg b.i.d. for 16 weeks, open-label 10 mg b.i.d. for 4 weeks, then variable 5 or 10 mg b.i.d. to Week 52. Primary end-points at Week 16 were the proportion of patients achieving at least a 75% reduction in Psoriasis Area and Severity Index (PASI75) and Physician's Global Assessment of "clear" or "almost clear" (PGA response) for psoriasis, and 20% or more improvement in American College of Rheumatology criteria (ACR20) for patients with psoriatic arthritis. Safety was assessed throughout. Eighty-seven patients met eligibility criteria for moderate to severe plaque psoriasis (5 mg b.i.d., n = 43; 10 mg b.i.d., n = 44), 12 met eligibility criteria for psoriatic arthritis (5 mg b.i.d., n = 4; 10 mg b.i.d., n = 8) including five who met both criteria (10 mg b.i.d.). At Week 16, 62.8% and 72.7% of patients achieved PASI75 with tofacitinib 5 and 10 mg b.i.d., respectively; 67.4% and 68.2% achieved PGA responses; all patients with psoriatic arthritis achieved ACR20. Responses were maintained through Week 52. Adverse events occurred in 83% of patients through Week 52, including four (4.3%) serious adverse events and three (3.2%) serious infections (all herpes zoster). No malignancies, cardiovascular events or deaths occurred. Tofacitinib (both doses) demonstrated efficacy in patients with moderate to severe plaque psoriasis and/or psoriatic arthritis through 52 weeks; safety findings were generally consistent with prior studies.
Keywords: Japan; kinase inhibitor; plaque psoriasis; psoriatic arthritis; tofacitinib.
© 2016 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures



References
-
- Gudjonsson JE, Elder JT. Psoriasis In: Goldsmith LA, Katz SI, Gilchrest BA, Paller A, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine, 8th edn New York: McGraw‐Hill Medical, 2012; 197–232.
-
- Schön MP, Boehncke WH. Psoriasis. N Engl J Med 2005; 352: 1899–1912. - PubMed
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41: 401–407. - PubMed
-
- Gelfand JM, Stern RS, Nijsten T et al The prevalence of psoriasis in African Americans: results from a population‐based study. J Am Acad Dermatol 2005; 52: 23–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

