Gut immunity in a protochordate involves a secreted immunoglobulin-type mediator binding host chitin and bacteria
- PMID: 26875669
- PMCID: PMC4757023
- DOI: 10.1038/ncomms10617
Gut immunity in a protochordate involves a secreted immunoglobulin-type mediator binding host chitin and bacteria
Abstract
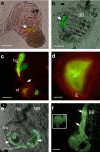
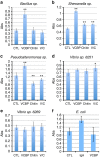
Protochordate variable region-containing chitin-binding proteins (VCBPs) consist of immunoglobulin-type V domains and a chitin-binding domain (CBD). VCBP V domains facilitate phagocytosis of bacteria by granulocytic amoebocytes; the function of the CBD is not understood. Here we show that the gut mucosa of Ciona intestinalis contains an extensive matrix of chitin fibrils to which VCBPs bind early in gut development, before feeding. Later in development, VCBPs and bacteria colocalize to chitin-rich mucus along the intestinal wall. VCBP-C influences biofilm formation in vitro and, collectively, the findings of this study suggest that VCBP-C may influence the overall settlement and colonization of bacteria in the Ciona gut. Basic relationships between soluble immunoglobulin-type molecules, endogenous chitin and bacteria arose early in chordate evolution and are integral to the overall function of the gut barrier.
Figures





Similar articles
-
A Soluble Immune Effector Binds Both Fungi and Bacteria via Separate Functional Domains.Front Immunol. 2019 Mar 6;10:369. doi: 10.3389/fimmu.2019.00369. eCollection 2019. Front Immunol. 2019. PMID: 30894858 Free PMC article.
-
A role for variable region-containing chitin-binding proteins (VCBPs) in host gut-bacteria interactions.Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16747-52. doi: 10.1073/pnas.1109687108. Epub 2011 Sep 19. Proc Natl Acad Sci U S A. 2011. PMID: 21930927 Free PMC article.
-
Expression of Ciona intestinalis variable region-containing chitin-binding proteins during development of the gastrointestinal tract and their role in host-microbe interactions.PLoS One. 2014 May 2;9(5):e94984. doi: 10.1371/journal.pone.0094984. eCollection 2014. PLoS One. 2014. PMID: 24788831 Free PMC article.
-
An Immune Effector System in the Protochordate Gut Sheds Light on Fundamental Aspects of Vertebrate Immunity.Results Probl Cell Differ. 2015;57:159-73. doi: 10.1007/978-3-319-20819-0_7. Results Probl Cell Differ. 2015. PMID: 26537381 Review.
-
Reflections on the Use of an Invertebrate Chordate Model System for Studies of Gut Microbial Immune Interactions.Front Immunol. 2021 Feb 25;12:642687. doi: 10.3389/fimmu.2021.642687. eCollection 2021. Front Immunol. 2021. PMID: 33717199 Free PMC article. Review.
Cited by
-
Autotaxin loss accelerates intestinal inflammation by suppressing TLR4-mediated immune responses.EMBO Rep. 2020 Oct 5;21(10):e49332. doi: 10.15252/embr.201949332. Epub 2020 Sep 1. EMBO Rep. 2020. PMID: 32875703 Free PMC article.
-
Evolutionary perspective on the hematopoietic system through a colonial chordate: allogeneic immunity and hematopoiesis.Curr Opin Immunol. 2020 Feb;62:91-98. doi: 10.1016/j.coi.2019.12.006. Epub 2020 Jan 16. Curr Opin Immunol. 2020. PMID: 31954962 Free PMC article. Review.
-
Cultivation of gut microorganisms of the marine ascidian Halocynthia roretzi reveals their potential roles in the environmental adaptation of their host.Mar Life Sci Technol. 2022 Apr 26;4(2):201-207. doi: 10.1007/s42995-022-00131-4. eCollection 2022 May. Mar Life Sci Technol. 2022. PMID: 37073224 Free PMC article.
-
A Soluble Immune Effector Binds Both Fungi and Bacteria via Separate Functional Domains.Front Immunol. 2019 Mar 6;10:369. doi: 10.3389/fimmu.2019.00369. eCollection 2019. Front Immunol. 2019. PMID: 30894858 Free PMC article.
-
Dietary Chitin Particles Called Mimetic Fungi Ameliorate Colitis in Toll-Like Receptor 2/CD14- and Sex-Dependent Manners.Infect Immun. 2019 Apr 23;87(5):e00006-19. doi: 10.1128/IAI.00006-19. Print 2019 Mar. Infect Immun. 2019. PMID: 30782858 Free PMC article.
References
-
- Cannon J. P., Haire R. N. & Litman G. W. Identification of diversified genes that contain immunoglobulin-like variable regions in a protochordate. Nat. Immunol. 3, 1200–1207 (2002). - PubMed
-
- Cannon J. P., Haire R. N., Schnitker N., Mueller M. G. & Litman G. W. Individual protochordates have unique immune-type receptor repertoires. Curr. Biol. 14, R465–R466 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

