Development of a light-regulated cell-recovery system for non-photosynthetic bacteria
- PMID: 26875863
- PMCID: PMC4753666
- DOI: 10.1186/s12934-016-0426-6
Development of a light-regulated cell-recovery system for non-photosynthetic bacteria
Abstract
Background: Recent advances in the understanding of photosensing in biological systems have enabled the use of photoreceptors as novel genetic tools. Exploiting various photoreceptors that cyanobacteria possess, a green light-inducible gene expression system was previously developed for the regulation of gene expression in cyanobacteria. However, the applications of cyanobacterial photoreceptors are not limited to these bacteria but are also available for non-photosynthetic microorganisms by the coexpression of a cyanobacterial chromophore with a cyanobacteria-derived photosensing system. An Escherichia coli-derived self-aggregation system based on Antigen 43 (Ag43) has been shown to induce cell self-aggregation of various bacteria by exogenous introduction of the Ag43 gene.
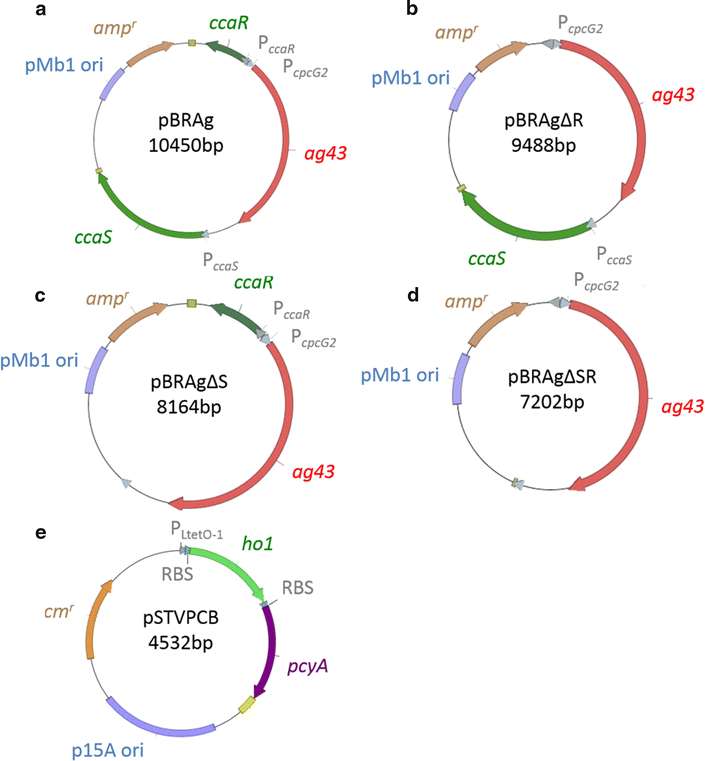
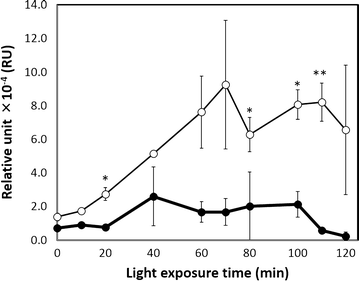
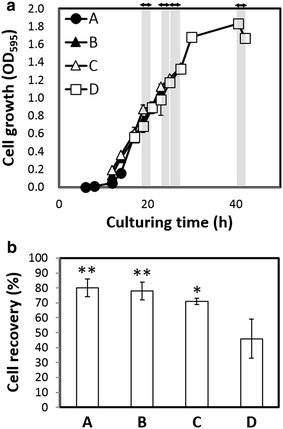
Results: An E. coli transformant harboring a plasmid encoding the Ag43 structural gene under a green light-regulated gene expression system derived from the cyanobacterium Synechocystis sp. PCC6803 was constructed. Ag43 was inserted downstream of the cpcG 2 promoter P cpcG2 , and its expression was regulated by green light induction, which was achieved by the functional expression of cyanobacterial CcaS/CcaR by coexpressing its chromophore synthesis gene cassette in E. coli. E. coli transformants harboring this designed system self-aggregated under green light exposure and precipitated, whereas transformants lacking the green light induction system did not. The green light induction system effectively functioned before the cell culture entered the stationary growth phase, and approximately 80 % of the cell culture was recovered by simple decantation.
Conclusion: This study demonstrated the construction of a cell recovery system for non-photosynthetic microorganisms induced by exposure of cells to green light. The system was regulated by a two-component regulatory system from cyanobacteria, and cell precipitation was mediated by an autotransporter protein, Ag43. Although further strict control and an increase of cell recovery efficiency are necessary, the system represents a novel tool for future bioprocessing with reduced energy and labor required for cell recovery.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

