Protein Allostery and Conformational Dynamics
- PMID: 26876046
- PMCID: PMC5011433
- DOI: 10.1021/acs.chemrev.5b00590
Protein Allostery and Conformational Dynamics
Abstract
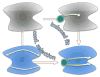
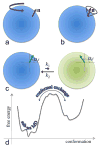
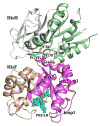
The functions of many proteins are regulated through allostery, whereby effector binding at a distal site changes the functional activity (e.g., substrate binding affinity or catalytic efficiency) at the active site. Most allosteric studies have focused on thermodynamic properties, in particular, substrate binding affinity. Changes in substrate binding affinity by allosteric effectors have generally been thought to be mediated by conformational transitions of the proteins or, alternatively, by changes in the broadness of the free energy basin of the protein conformational state without shifting the basin minimum position. When effector binding changes the free energy landscape of a protein in conformational space, the change affects not only thermodynamic properties but also dynamic properties, including the amplitudes of motions on different time scales and rates of conformational transitions. Here we assess the roles of conformational dynamics in allosteric regulation. Two cases are highlighted where NMR spectroscopy and molecular dynamics simulation have been used as complementary approaches to identify residues possibly involved in allosteric communication. Perspectives on contentious issues, for example, the relationship between picosecond-nanosecond local and microsecond-millisecond conformational exchange dynamics, are presented.
Figures

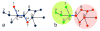

References
-
- Monod J, Jacob F. General Conclusions: Telenomic Mechanisms in Cellular Metabolism, Growth, and Differentiation. Cold Spring Harbor Symp Quant Biol. 1961;26:389–401. - PubMed
-
- Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Wyman J, Allen DW. The Problem of the Heme Interactions in Hemoglobin and the Basis of the Bohr Effect. J Polym Sci. 1951;7:499–518.
-
- Cooper A, Dryden DT. Allostery without Conformational Change. A Plausible Model. Eur Biophys J. 1984;11:103–109. - PubMed
-
- Nussinov R, Tsai CJ. Allostery without a Conformational Change? Revisiting the Paradigm. Curr Opin Struct Biol. 2015;30:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

