The C. elegans CCAAT-Enhancer-Binding Protein Gamma Is Required for Surveillance Immunity
- PMID: 26876169
- PMCID: PMC4767654
- DOI: 10.1016/j.celrep.2016.01.055
The C. elegans CCAAT-Enhancer-Binding Protein Gamma Is Required for Surveillance Immunity
Abstract
Pathogens attack host cells by deploying toxins that perturb core host processes. Recent findings from the nematode C. elegans and other metazoans indicate that surveillance or "effector-triggered" pathways monitor functioning of these core processes and mount protective responses when they are perturbed. Despite a growing number of examples of surveillance immunity, the signaling components remain poorly defined. Here, we show that CEBP-2, the C. elegans ortholog of mammalian CCAAT-enhancer-binding protein gamma, is a key player in surveillance immunity. We show that CEBP-2 acts together with the bZIP transcription factor ZIP-2 in the protective response to translational block by P. aeruginosa Exotoxin A as well as perturbations of other processes. CEBP-2 serves to limit pathogen burden, promote survival upon P. aeruginosa infection, and also promote survival upon Exotoxin A exposure. These findings may have broad implications for the mechanisms by which animals sense pathogenic attack and mount protective responses.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
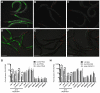
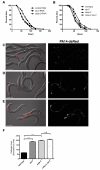
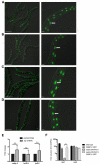
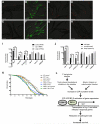
Similar articles
-
Tribbles ortholog NIPI-3 and bZIP transcription factor CEBP-1 regulate a Caenorhabditis elegans intestinal immune surveillance pathway.BMC Biol. 2016 Dec 7;14(1):105. doi: 10.1186/s12915-016-0334-6. BMC Biol. 2016. PMID: 27927200 Free PMC article.
-
Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans.Cell Host Microbe. 2012 Apr 19;11(4):364-74. doi: 10.1016/j.chom.2012.02.007. Cell Host Microbe. 2012. PMID: 22520464 Free PMC article.
-
C. elegans detects pathogen-induced translational inhibition to activate immune signaling.Cell Host Microbe. 2012 Apr 19;11(4):375-86. doi: 10.1016/j.chom.2012.02.008. Cell Host Microbe. 2012. PMID: 22520465 Free PMC article.
-
Local and long-range activation of innate immunity by infection and damage in C. elegans.Curr Opin Immunol. 2016 Feb;38:1-7. doi: 10.1016/j.coi.2015.09.005. Epub 2015 Oct 28. Curr Opin Immunol. 2016. PMID: 26517153 Review.
-
Strength in numbers: "Omics" studies of C. elegans innate immunity.Virulence. 2012 Oct 1;3(6):477-84. doi: 10.4161/viru.21906. Epub 2012 Oct 1. Virulence. 2012. PMID: 23076279 Free PMC article. Review.
Cited by
-
The transcription factor ZIP-1 promotes resistance to intracellular infection in Caenorhabditis elegans.Nat Commun. 2022 Jan 10;13(1):17. doi: 10.1038/s41467-021-27621-w. Nat Commun. 2022. PMID: 35013162 Free PMC article.
-
Tribbles ortholog NIPI-3 and bZIP transcription factor CEBP-1 regulate a Caenorhabditis elegans intestinal immune surveillance pathway.BMC Biol. 2016 Dec 7;14(1):105. doi: 10.1186/s12915-016-0334-6. BMC Biol. 2016. PMID: 27927200 Free PMC article.
-
Patterns of pathogenesis in innate immunity: insights from C. elegans.Nat Rev Immunol. 2025 Apr 17. doi: 10.1038/s41577-025-01167-0. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40247006 Review.
-
Surveillance Immunity: An Emerging Paradigm of Innate Defense Activation in Caenorhabditis elegans.PLoS Pathog. 2016 Sep 15;12(9):e1005795. doi: 10.1371/journal.ppat.1005795. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27631629 Free PMC article. Review. No abstract available.
-
Innate Immunity in the C. elegans Intestine Is Programmed by a Neuronal Regulator of AWC Olfactory Neuron Development.Cell Rep. 2020 Apr 7;31(1):107478. doi: 10.1016/j.celrep.2020.03.042. Cell Rep. 2020. PMID: 32268082 Free PMC article.
References
-
- Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nature immunology. 2005;6:973–979. - PubMed
-
- Casas V, Miyake J, Balsley H, Roark J, Telles S, Leeds S, Zurita I, Breitbart M, Bartlett D, Azam F, et al. Widespread occurrence of phage-encoded exotoxin genes in terrestrial and aquatic environments in Southern California. FEMS Microbiol Lett. 2006;261:141–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

