Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway
- PMID: 26876200
- PMCID: PMC5028421
- DOI: 10.1038/onc.2016.23
Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway
Abstract
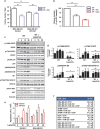
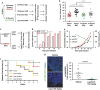
In breast cancer (BC) patients, local recurrences often arise in proximity of the surgical scar, suggesting that response to surgery may have a causative role. Radiotherapy (RT) after lumpectomy significantly reduces the risk of recurrence. We investigated the direct effects of surgery and of RT delivered intraoperatively (IORT), by collecting irradiated and non-irradiated breast tissues from BC patients, after tumor removal. These breast tissue specimens have been profiled for their microRNA (miR) expression, in search of differentially expressed miR among patients treated or not with IORT. Our results demonstrate that IORT elicits effects that go beyond the direct killing of residual tumor cells. IORT altered the wound response, inducing the expression of miR-223 in the peri-tumoral breast tissue. miR-223 downregulated the local expression of epidermal growth factor (EGF), leading to decreased activation of EGF receptor (EGFR) on target cells and, eventually, dampening a positive EGF-EGFR autocrine/paracrine stimulation loop induced by the post-surgical wound-healing response. Accordingly, both RT-induced miR-223 and peri-operative inhibition of EGFR efficiently prevented BC cell growth and reduced recurrence formation in mouse models of BC. Our study uncovers unknown effects of RT delivered on a wounded tissue and prompts to the use of anti-EGFR treatments, in a peri-operative treatment schedule, aimed to timely treat BC patients and restrain recurrence formation.
Figures

References
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659. - PubMed
-
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 2012; 12: 170–180. - PubMed
-
- Baum M, Demicheli R, Hrushesky W, Retsky M. Does surgery unfavourably perturb the ‘natural history' of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 2005; 41: 508–515. - PubMed
-
- Belletti B, Vaidya JS, D'Andrea S, Entschladen F, Roncadin M, Lovat F et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008; 14: 1325–1332. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

