Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression
- PMID: 26876208
- PMCID: PMC4985510
- DOI: 10.1038/onc.2016.25
Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression
Abstract
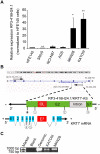
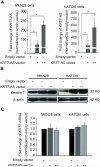
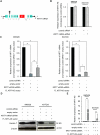
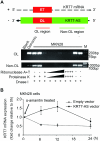
Alterations in long non-coding RNAs (lncRNAs) are associated with human carcinogenesis. One group of lncRNAs, which are antisense in orientation to coding mRNAs (ASs), have been recently described in cancers but are poorly understood. We sought to identify ASs involved in human gastric cancer (GC) and to elucidate their mechanisms of action in carcinogenesis. We performed massively parallel RNA sequencing in GCs and matched normal tissues, as well as in GC-derived and normal gastric epithelial cell lines. One AS, designated Homo sapiens keratin 7 (KRT7-AS), was selected due to its marked upregulation and concordant expression with its cognate sense counterpart, KRT7, in GC tissues and cell lines. KRT7-AS formed an RNA-RNA hybrid with KRT7 and controlled KRT7 expression at both the mRNA and the post-transcriptional levels. Moreover, forced overexpression of the KRT7-overlapping region (OL) of KRT7-AS (but not its non-KRT7-OL portions) increased keratin 7 protein levels in cells. Finally, forced overexpression of full-length KRT7-AS or OL KRT7-AS (but not its non-KRT7-OL regions) promoted GC cell proliferation and migration. We conclude that lncRNA KRT7-AS promotes GC, at least in part, by increasing KRT7 expression.
Figures



Similar articles
-
The long non-coding RNA keratin-7 antisense acts as a new tumor suppressor to inhibit tumorigenesis and enhance apoptosis in lung and breast cancers.Cell Death Dis. 2023 Apr 25;14(4):293. doi: 10.1038/s41419-023-05802-3. Cell Death Dis. 2023. PMID: 37185462 Free PMC article.
-
Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7.Gut Microbes. 2020 May 3;11(3):511-525. doi: 10.1080/19490976.2019.1695494. Epub 2020 Jan 7. Gut Microbes. 2020. PMID: 31910722 Free PMC article.
-
Identification of novel biomarkers, MUC5AC, MUC1, KRT7, GAPDH, CD44 for gastric cancer.Med Oncol. 2020 Mar 27;37(5):34. doi: 10.1007/s12032-020-01362-0. Med Oncol. 2020. PMID: 32219571
-
The functional sites of miRNAs and lncRNAs in gastric carcinogenesis.Tumour Biol. 2015 Feb;36(2):521-32. doi: 10.1007/s13277-015-3136-5. Epub 2015 Feb 1. Tumour Biol. 2015. PMID: 25636450 Free PMC article. Review.
-
Long noncoding RNAs: novel insights into gastric cancer.Cancer Lett. 2015 Jan 28;356(2 Pt B):357-66. doi: 10.1016/j.canlet.2014.11.005. Epub 2014 Nov 7. Cancer Lett. 2015. PMID: 25444905 Review.
Cited by
-
Long Non-coding Antisense RNA TNRC6C-AS1 Is Activated in Papillary Thyroid Cancer and Promotes Cancer Progression by Suppressing TNRC6C Expression.Front Endocrinol (Lausanne). 2018 Jul 9;9:360. doi: 10.3389/fendo.2018.00360. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30038597 Free PMC article.
-
Identification of pyroptosis-related subtypes, development of a prognostic model, and characterization of tumour microenvironment infiltration in gastric cancer.Front Genet. 2022 Jul 18;13:963565. doi: 10.3389/fgene.2022.963565. eCollection 2022. Front Genet. 2022. PMID: 35923703 Free PMC article.
-
Post-transcriptional Regulation of Genes Related to Biological Behaviors of Gastric Cancer by Long Noncoding RNAs and MicroRNAs.J Cancer. 2017 Nov 12;8(19):4141-4154. doi: 10.7150/jca.22076. eCollection 2017. J Cancer. 2017. PMID: 29187891 Free PMC article. Review.
-
Biological and clinical relevance of correlated expression levels of coding and long noncoding RNAs in HPV16 positive cervical cancers.Hum Genomics. 2024 Aug 29;18(1):91. doi: 10.1186/s40246-024-00660-2. Hum Genomics. 2024. PMID: 39210444 Free PMC article.
-
RP11-867G2.8 promotes EMT and chordoma malignant phenotypes by enhancing FUT4 mRNA stability and translation.Am J Cancer Res. 2022 Mar 15;12(3):1264-1281. eCollection 2022. Am J Cancer Res. 2022. PMID: 35411246 Free PMC article.
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–2917. - PubMed
-
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. - PubMed
-
- Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2:88–94. - PubMed
-
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. - PubMed
-
- Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, Nakamura M, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

