Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer
- PMID: 26876480
- PMCID: PMC4753416
- DOI: 10.1038/srep21225
Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer
Abstract
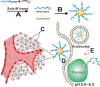
Ample attention has focused on cancer drug delivery via prodrug nanoparticles due to their high drug loading property and comparatively lower side effects. In this study, we designed a PEG-DOX-Cur prodrug nanoparticle for simultaneous delivery of doxorubicin (DOX) and curcumin (Cur) as a combination therapy to treat cancer. DOX was conjugated to PEG by Schiff's base reaction. The obtained prodrug conjugate could self-assemble in water at pH 7.4 into nanoparticles (PEG-DOX NPs) and encapsulate Cur into the core through hydrophobic interaction (PEG-DOX-Cur NPs). When the PEG-DOX-Cur NPs are internalized by tumor cells, the Schiff's base linker between PEG and DOX would break in the acidic environment that is often observed in tumors, causing disassembling of the PEG-DOX-Cur NPs and releasing both DOX and Cur into the nuclei and cytoplasma of the tumor cells, respectively. Compared with free DOX, free Cur, free DOX-Cur combination, or PEG-DOX NPs, PEG-DOX-Cur NPs exhibited higher anti-tumor activity in vitro. In addition, the PEG-DOX-Cur NPs also showed prolonged blood circulation time, elevated local drug accumulation and increased tumor penetration. Enhanced anti-tumor activity was also observed from the PEG-DOX-Cur-treated animals, demonstrating better tumor inhibitory property of the NPs. Thus, the PEG-DOX-Cur prodrug nanoparticle system provides a simple yet efficient approach of drug delivery for chemotherapy.
Figures









References
-
- Huang P. et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 136, 11748–11756, (2014). - PubMed
-
- Duan X. et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS nano 7, 5858–5869, (2013). - PubMed
-
- Podder H. et al. Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J. Am. Soc. Neohrol. 12, 1059–1071 (2001). - PubMed
-
- Mayer L. D. et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol. Cancer. Ther. 5, 1854–1863, (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

