Liraglutide Compromises Pancreatic β Cell Function in a Humanized Mouse Model
- PMID: 26876561
- PMCID: PMC4785083
- DOI: 10.1016/j.cmet.2016.01.009
Liraglutide Compromises Pancreatic β Cell Function in a Humanized Mouse Model
Abstract
Incretin mimetics are frequently used in the treatment of type 2 diabetes because they potentiate β cell response to glucose. Clinical evidence showing short-term benefits of such therapeutics (e.g., liraglutide) is abundant; however, there have been several recent reports of unexpected complications in association with incretin mimetic therapy. Importantly, clinical evidence on the potential effects of such agents on the β cell and islet function during long-term, multiyear use remains lacking. We now show that prolonged daily liraglutide treatment of >200 days in humanized mice, transplanted with human pancreatic islets in the anterior chamber of the eye, is associated with compromised release of human insulin and deranged overall glucose homeostasis. These findings raise concern about the chronic potentiation of β cell function through incretin mimetic therapy in diabetes.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
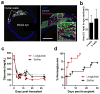
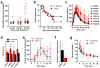
Comment in
-
Diabetes: Concerns about long-term use of GLP-1 analogues.Nat Rev Endocrinol. 2016 Apr;12(4):186. doi: 10.1038/nrendo.2016.33. Epub 2016 Mar 4. Nat Rev Endocrinol. 2016. PMID: 26939977 No abstract available.
References
-
- Glucose-lowering treatment of type 2 diabetes. Part II--Glucose-lowering drugs after metformin: a choice based largely on adverse effects. Prescrire Int. 2015;24:130–135. No authors listed. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

