The TCA cycle transferase DLST is important for MYC-mediated leukemogenesis
- PMID: 26876595
- PMCID: PMC4889531
- DOI: 10.1038/leu.2016.26
The TCA cycle transferase DLST is important for MYC-mediated leukemogenesis
Abstract
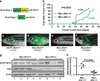
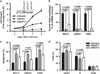
Despite the pivotal role of MYC in the pathogenesis of T-cell acute lymphoblastic leukemia (T-ALL) and many other cancers, the mechanisms underlying MYC-mediated tumorigenesis remain inadequately understood. Here we utilized a well-characterized zebrafish model of Myc-induced T-ALL for genetic studies to identify novel genes contributing to disease onset. We found that heterozygous inactivation of a tricarboxylic acid (TCA) cycle enzyme, dihydrolipoamide S-succinyltransferase (Dlst), significantly delayed tumor onset in zebrafish without detectable effects on fish development. DLST is the E2 transferase of the α-ketoglutarate (α-KG) dehydrogenase complex (KGDHC), which converts α-KG to succinyl-CoA in the TCA cycle. RNAi knockdown of DLST led to decreased cell viability and induction of apoptosis in human T-ALL cell lines. Polar metabolomics profiling revealed that the TCA cycle was disrupted by DLST knockdown in human T-ALL cells, as demonstrated by an accumulation of α-KG and a decrease of succinyl-CoA. Addition of succinate, the downstream TCA cycle intermediate, to human T-ALL cells was sufficient to rescue defects in cell viability caused by DLST inactivation. Together, our studies uncovered an important role for DLST in MYC-mediated leukemogenesis and demonstrated the metabolic dependence of T-lymphoblasts on the TCA cycle, thus providing implications for targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


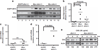


References
-
- Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. - PubMed
-
- Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. - PubMed
-
- Koch U, Radtke F. Notch in T-ALL: new players in a complex disease. Trends Immunol. 2011;32:434–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

