An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering
- PMID: 26876685
- PMCID: PMC4753477
- DOI: 10.1038/srep21021
An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering
Abstract
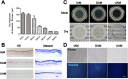
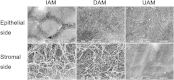
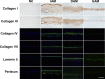
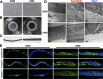
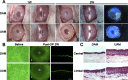
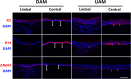
Amniotic membranes (AMs) are widely used as a corneal epithelial tissue carrier in reconstruction surgery. However, the engineered tissue transparency is low due to the translucent thick underlying AM stroma. To overcome this drawback, we developed an ultra-thin AM (UAM) by using collagenase IV to strip away from the epithelial denuded AM (DAM) some of the stroma. By thinning the stroma to about 30 μm, its moist and dry forms were rendered acellular, optically clear and its collagen framework became compacted and inerratic. Engineered rabbit corneal epithelial cell (RCEC) sheets generated through expansion of limbal epithelial cells on UAM were more transparent and thicker than those expanded on DAM. Moreover, ΔNp63 and ABCG2 gene expression was greater in tissue engineered cell sheets expanded on UAM than on DAM. Furthermore, 2 weeks after surgery, the cornea grafted with UAM based cell sheets showed higher transparency and more stratified epithelium than the cornea grafted with DAM based cell sheets. Taken together, tissue engineered corneal epithelium generated on UAM has a preferable outcome because the transplanted tissue is more transparent and better resembles the phenotype of the native tissue than that obtained by using DAM for this procedure. UAM preserves compact layer of the amniotic membrane and maybe an ideal substrate for corneal epithelial tissue engineering.
Figures


Similar articles
-
A novel tissue-engineered corneal epithelium based on ultra-thin amniotic membrane and mesenchymal stem cells.Sci Rep. 2024 Jul 29;14(1):17407. doi: 10.1038/s41598-024-68219-8. Sci Rep. 2024. PMID: 39075142 Free PMC article.
-
Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier.Invest Ophthalmol Vis Sci. 2007 Feb;48(2):597-604. doi: 10.1167/iovs.06-0664. Invest Ophthalmol Vis Sci. 2007. PMID: 17251455
-
Cultured corneal epithelia for ocular surface disease.Trans Am Ophthalmol Soc. 1999;97:891-986. Trans Am Ophthalmol Soc. 1999. PMID: 10703147 Free PMC article.
-
Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction.Exp Eye Res. 2004 Mar;78(3):483-91. doi: 10.1016/j.exer.2003.09.004. Exp Eye Res. 2004. PMID: 15106927 Review.
-
Recent advances in cultivated epithelial transplantation.Cornea. 2008 Sep;27 Suppl 1:S41-7. doi: 10.1097/ICO.0b013e31817f358e. Cornea. 2008. PMID: 18813074 Review.
Cited by
-
A novel tissue-engineered corneal epithelium based on ultra-thin amniotic membrane and mesenchymal stem cells.Sci Rep. 2024 Jul 29;14(1):17407. doi: 10.1038/s41598-024-68219-8. Sci Rep. 2024. PMID: 39075142 Free PMC article.
-
General consensus on multimodal functions and validation analysis of perinatal derivatives for regenerative medicine applications.Front Bioeng Biotechnol. 2022 Oct 3;10:961987. doi: 10.3389/fbioe.2022.961987. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36263355 Free PMC article. Review.
-
Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds.Membranes (Basel). 2021 Nov 29;11(12):941. doi: 10.3390/membranes11120941. Membranes (Basel). 2021. PMID: 34940442 Free PMC article. Review.
-
Development of a tissue-engineered skin substitute on a base of human amniotic membrane.J Tissue Eng. 2019 Feb 2;10:2041731418825378. doi: 10.1177/2041731418825378. eCollection 2019 Jan-Dec. J Tissue Eng. 2019. PMID: 30746119 Free PMC article.
-
Corneal Repair and Regeneration: Current Concepts and Future Directions.Front Bioeng Biotechnol. 2019 Jun 11;7:135. doi: 10.3389/fbioe.2019.00135. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31245365 Free PMC article. Review.
References
-
- Pellegrini G., Rama P., Di Rocco A., Panaras A. & De Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem cells 32, 26–34 (2014). - PubMed
-
- Grueterich M., Espana E. M. & Tseng S. C. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Sur Ophthalmol 48, 631–646 (2003). - PubMed
-
- Ramaesh K. & Dhillon B. Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. Eur J Ophthalmol 13, 515–524 (2003). - PubMed
-
- Shortt A. J., Tuft S. J. & Daniels J. T. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf 8, 80–90 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

