Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps
- PMID: 26876876
- PMCID: PMC4829450
- DOI: 10.1016/j.actbio.2016.02.017
Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps
Abstract
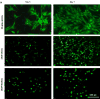
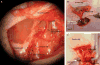
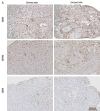
Using a perfusion decellularization protocol, we developed a decellularized skin/adipose tissue flap (DSAF) comprising extracellular matrix (ECM) and intact vasculature. Our DSAF had a dominant vascular pedicle, microcirculatory vascularity, and a sensory nerve network and retained three-dimensional (3D) nanofibrous structures well. DSAF, which was composed of collagen and laminin with well-preserved growth factors (e.g., vascular endothelial growth factor, basic fibroblast growth factor), was successfully repopulated with human adipose-derived stem cells (hASCs) and human umbilical vein endothelial cells (HUVECs), which integrated with DSAF and formed 3D aggregates and vessel-like structures in vitro. We used microsurgery techniques to re-anastomose the recellularized DSAF into nude rats. In vivo, the engineered flap construct underwent neovascularization and constructive remodeling, which was characterized by the predominant infiltration of M2 macrophages and significant adipose tissue formation at 3months postoperatively. Our results indicate that DSAF co-cultured with hASCs and HUVECs is a promising platform for vascularized soft tissue flap engineering. This platform is not limited by the flap size, as the entire construct can be immediately perfused by the recellularized vascular network following simple re-integration into the host using conventional microsurgical techniques.
Statement of significance: Significant soft tissue loss resulting from traumatic injury or tumor resection often requires surgical reconstruction using autologous soft tissue flaps. However, the limited availability of qualitative autologous flaps as well as the donor site morbidity significantly limits this approach. Engineered soft tissue flap grafts may offer a clinically relevant alternative to the autologous flap tissue. In this study, we engineered vascularized soft tissue free flap by using skin/adipose flap extracellular matrix scaffold (DSAF) in combination with multiple types of human cells. Following vascular reanastomosis in the recipient site, the engineered products successful regenerated large-scale fat tissue in vivo. This approach may provide a translatable platform for composite soft tissue free flap engineering for microsurgical reconstruction.
Keywords: Adipose tissue engineering; Decellularization; Extracellular matrix scaffold; Skin/adipose tissue flap matrix; Soft tissue flap engineering; Vascularized composite tissue flap engineering.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





















References
-
- Shores JT, Brandacher G, Lee WP. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg. 2015;135:351e–60e. - PubMed
-
- Kueckelhaus M, Fischer S, Seyda M, Bueno EM, Aycart MA, Alhefzi M, ElKhal A, Pomahac B, Tullius SG. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl Int. 2015 Aug 12; doi: 10.1111/tri.12652. [Epub ahead of print] - DOI - PMC - PubMed
-
- Siemionow M, Nasir S. Chimerism and bone marrow based therapies in transplantation. Microsurgery. 2007;27:510–21. - PubMed
-
- Siemionow M, Ortak T, Izycki D, Oke R, Cunningham B, Prajapati R, Zins JE. Induction of tolerance in composite-tissue allografts. Transplantation. 2002;74:1211–7. - PubMed
-
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

