"Nanofiltration" Enabled by Super-Absorbent Polymer Beads for Concentrating Microorganisms in Water Samples
- PMID: 26876979
- PMCID: PMC4753426
- DOI: 10.1038/srep20516
"Nanofiltration" Enabled by Super-Absorbent Polymer Beads for Concentrating Microorganisms in Water Samples
Abstract
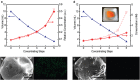
Detection and quantification of pathogens in water is critical for the protection of human health and for drinking water safety and security. When the pathogen concentrations are low, large sample volumes (several liters) are needed to achieve reliable quantitative results. However, most microbial identification methods utilize relatively small sample volumes. As a consequence, a concentration step is often required to detect pathogens in natural waters. Herein, we introduce a novel water sample concentration method based on superabsorbent polymer (SAP) beads. When SAP beads swell with water, small molecules can be sorbed within the beads, but larger particles are excluded and, thus, concentrated in the residual non-sorbed water. To illustrate this approach, millimeter-sized poly(acrylamide-co-itaconic acid) (P(AM-co-IA)) beads are synthesized and successfully applied to concentrate water samples containing two model microorganisms: Escherichia coli and bacteriophage MS2. Experimental results indicate that the size of the water channel within water swollen P(AM-co-IA) hydrogel beads is on the order of several nanometers. The millimeter size coupled with a negative surface charge of the beads are shown to be critical in order to achieve high levels of concentration. This new concentration procedure is very fast, effective, scalable, and low-cost with no need for complex instrumentation.
Figures



Similar articles
-
Synthesis and application of superabsorbent polymer microspheres for rapid concentration and quantification of microbial pathogens in ambient water.Sep Purif Technol. 2020 May 15;239:116540. doi: 10.1016/j.seppur.2020.116540. Sep Purif Technol. 2020. PMID: 32421015 Free PMC article.
-
GAC adsorption filters as barriers for viruses, bacteria and protozoan (oo)cysts in water treatment.Water Res. 2010 Feb;44(4):1224-34. doi: 10.1016/j.watres.2009.10.011. Epub 2009 Nov 4. Water Res. 2010. PMID: 19892384
-
Assessment of poly-L-lysine dendrigrafts for virus concentration in water: use of MS2 bacteriophage as proof of concept.J Appl Microbiol. 2013 Jul;115(1):290-7. doi: 10.1111/jam.12209. Epub 2013 Apr 19. J Appl Microbiol. 2013. PMID: 23551794
-
A mathematical model for removal of human pathogenic viruses and bacteria by slow sand filtration under variable operational conditions.Water Res. 2013 May 1;47(7):2592-602. doi: 10.1016/j.watres.2013.02.027. Epub 2013 Feb 27. Water Res. 2013. PMID: 23490102
-
Synthesis and characterization of thermo- and pH-sensitive poly(vinyl alcohol)/poly(N, N-diethylacrylamide-co-itaconic acid) semi-IPN hydrogels.Biomed Mater. 2012 Jun;7(3):035014. doi: 10.1088/1748-6041/7/3/035014. Epub 2012 Apr 11. Biomed Mater. 2012. PMID: 22493167
Cited by
-
Single-step equipment-free extracellular vesicle concentration using super absorbent polymer beads.J Extracell Vesicles. 2021 Feb;10(4):e12074. doi: 10.1002/jev2.12074. Epub 2021 Feb 23. J Extracell Vesicles. 2021. PMID: 33664938 Free PMC article.
-
Self-Driven "Microfiltration" Enabled by Porous Superabsorbent Polymer (PSAP) Beads for Biofluid Specimen Processing and Storage.ACS Mater Lett. 2020 Nov 2;2(11):1545-1554. doi: 10.1021/acsmaterialslett.0c00348. Epub 2020 Oct 21. ACS Mater Lett. 2020. PMID: 33163968 Free PMC article.
-
Superabsorbent Polymers as a Soil Amendment for Increasing Agriculture Production with Reducing Water Losses under Water Stress Condition.Polymers (Basel). 2022 Dec 29;15(1):161. doi: 10.3390/polym15010161. Polymers (Basel). 2022. PMID: 36616513 Free PMC article. Review.
-
Development of a proof-of-concept microfluidic portable pathogen analysis system for water quality monitoring.Sci Total Environ. 2022 Mar 20;813:152556. doi: 10.1016/j.scitotenv.2021.152556. Epub 2021 Dec 21. Sci Total Environ. 2022. PMID: 34952082 Free PMC article.
-
Improving Isolation of Extracellular Vesicles by Utilizing Nanomaterials.Membranes (Basel). 2021 Dec 31;12(1):55. doi: 10.3390/membranes12010055. Membranes (Basel). 2021. PMID: 35054584 Free PMC article. Review.
References
-
- Khasnis A. A. & Nettleman M. D. Global warming and infectious disease. Arch. Med. Res. 36, 689–696 (2005). - PubMed
-
- Mathers C. D., Sadana R., Salomon J. A., Murray C. J. L. & Lopez A. D. Healthy life expectancy in 191 countries, 1999. Lancet 357, 1685–1691 (2001). - PubMed
-
- Shannon M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). - PubMed
-
- Sales-Ortells H., Agostini G. & Medema G. Quantification of waterborne pathogens and associated health risks in urban water. Environ. Sci. Technol. 49, 6943–6952 (2015). - PubMed
-
- Bhutta Z. A. Polio eradication hinges on child health in Pakistan. Nature 511, 285–287 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

